Prediction and Discovery
Science is often portrayed as a fixed set of rules, impersonal, and devoid of emotions. It is not, as can be seen from the discovery of fullerenes.
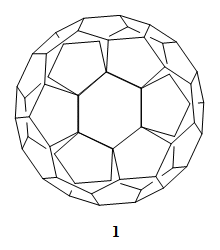
The best known fullerene, C60 (1), is a molecule of a very high symmetry, Ih. This means that all the 60 carbon atoms are equivalent, and by starting from one of them every other one can be reached by applying symmetry operations. In his famous book “The Infrared and Raman Spectra of Polyatomic Molecules” from 1945, future Nobel Prize winner, Gerhard Herzberg (1971), predicted: “It is not likely that molecules of such a symmetry will ever be found.” [1]
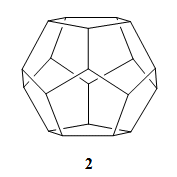
However, in 1981, Leo Paquette’s group, Ohio State University, Columbus, USA, described the synthesis of a regular dodecahedrane (2) with all twenty carbon atoms equivalent [2]. It took a little longer to obtain C60 (1), of regular icosahedral symmetry. The molecule had been proposed in work by Japanese researchers in 1970 [3], and the calculations for it were published in Russian by Soviet scientists in 1973 [4].
For several years no one was interested in these works. Then, in 1984, the mass spectrum of carbon clusters was published [5], in which there was a strong signal corresponding to C60. The authors published the whole spectrum, but they were not interested in the C60 signal, because they were only involved in studies of small carbon clusters and so the Nobel Prize went to someone else.
The next year, the recognized specialist in astrochemistry, i.e., the science of chemistry in space, Professor Harold Kroto, then at the University of Sussex, UK, visited Professors Robert Curl and Richard Smalley at Rice University, Texas, USA, planning to carry out a joint study of products resulting from discharges in a vacuum tube containing carbon black. In this experiment, developed to mimic conditions in space, a “forest” of signals was obtained in the mass spectrum of the products with the strongest one appearing at e/m = 720. A student, who was asked to optimize the experimental conditions, obtained a spectrum consisting of only two peaks: one strong signal corresponding to e/m = 720, that is to C60, and a weaker one corresponding to C70 (3) [6]. Mass spectra allow one to determine only the molecular formula, C60 in this case, however, it was very difficult to assign the structure.
Possible Structures and Structure Assignment
Various possible structures were considered for C60, among others were those with so called “dangling bonds” (Fig. 1), that is condensed aromatic ring systems lying one above the other having single bonds without any substituents.
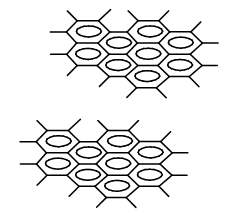
Figure 1. A hypothetical model of the structure of C60 with dangling single bonds bearing no substituents at the ends.
Were graphics not Kroto’s hobby and without his knowledge of the geodesmic domes by architect Buckminster Fuller, the search for the structure of C60 would definitely have lasted much longer. Incidentally, Fuller, who later had great achievements in several fields and was an honorary doctor of several universities, in his youth considered himself very unlucky: at around the age of 30 he was “Jobless, without savings or prospects, with a wife and newborn daughter to support, suicidal and drinking heavily” [7].
It is amazing that Kroto, Smalley, and Curl did not know that the high symmetry formula they proposed had a very common, well-known shape. The next morning they called the Department of Mathematics at Rice University, only to find out that this was simply a football or soccer ball. The entire story is attractively described by Kroto in a review [8].
It should be noted that in the same year in which C60 was discovered, the first fullerene endohedral complex (i.e., a fullerene cage enclosing one or more ions or other particles or molecules, of the same type as (4)) was obtained [9].
Only trace amounts of fullerene could be obtained until 1990, and its structure could not be proven. The breakthrough was a procedure for obtaining large fullerene quantities and their purification proposed by Wolfgang Krätschmer, Max-Planck Institute for Nuclear Physics, Heidelberg, Germany, and collaborators [10]. This allowed Kroto to isolate macroscopic quantities of C60 and C70 and elucidate their spatial structure on the basis of 13C NMR spectra. As expected, only one signal for the first molecule and the five signals for the other were observed in the spectrum, which clearly confirmed earlier hypotheses concerning their structure [11].
At present NMR techniques are often used to explain the structure of organic compounds, but the interpretation of spectra is usually much more complicated than in the case of highly symmetrical C60 and C70.
Even more remarkable, the NMR method is used to measure bond lengths. Although all atoms in C60 are equivalent, there are two different types of bonds in the molecule: those connecting two six-membered rings and those connecting five-membered rings to six-membered ones. Their length could not be measured in the first X-ray analysis carried out at room temperature. Due to the shape of the almost spherical C60 cage and thermal vibrations at room temperature, the study only allowed to establish the cage diameter and the distance between the cages. Then, by measuring the chemical shift anisotropy at low temperature with 13C NMR spectroscopy, Costantino Yannoni’s group, IBM Research Division, San Jose, California, USA, was able to determine the length of the bonds in the molecule [12].
Definitely, NMR spectroscopy is not the best way to determine the interatomic distance, so the measurement accuracy was low. However, this method clearly shows that in C60 there are two types of bonds with different lengths.
Many Promising Applications
With the discovery of fullerenes, the “applications rush” began. The problem deserves a more detailed discussion which will be presented later. Let us just mention here that a few years after the discovery of fullerenes, both scientific journals and newspapers started to swarm with proposals of fullerene applications and, especially, of their endohedral complexes. For many years, very few of these promises could be realized. The same is true for carbon nanotubes, similar to, but usually much longer than 5.
In 2000, researchers at IBM announced that in ten years electronics on the industrial scale would be based on carbon nanotubes not on silicon. As we can see, nothing has come of it yet, and now the history repeats itself with graphene. The only large-sale marketed applications of fullerenes and carbon nanotubes are as additives for improving, to a certain extent, the properties of materials.
Fullerenes, carbon nanotubes, and graphene are fascinating systems with very unusual properties. Certainly they are worth detailed and multifarious research. However, to expect that they soon will bring significant multiscale implementations is incorrect. For those we will have to wait much longer.
As you can see the discovery of fullerenes was neither quick nor easy, and rich in surprising twists. Of no importance, but interesting for me as a Pole: because of the name, I thought for a long time that Kroto is Japanese. In fact, Sir Harold Walter Kroto was born in England, and the Kroto name is an abbreviation of the one worn by his parents, Polish Jews, Krotoszyńscy.
References
[1] G. Herzberg, Infrared and Raman Spectra of Polyatomic Molecules, Lancaster Press, Lancaster, UK, 1945. ISBN: 978-0894642692
[2] R. J. Ternansky, D. W. Balogh, L. Paquette, J. Am. Chem. Soc. 1982, 104, 4503. DOI: 10.1021/ja00380a040
[3] E. Osawa, Kagaku, Kyoto, Japan, 1970, 25, 854.
[4] D. E. Botchvar, E. G. Galpern, Dokl. AN SSSR 1973, 209, 610.
[5] E. A. Rohlfing, D. M. Cox, A. Kaldor, J. Chem. Phys. 1984, 81, 3322. DOI: 10.1063/1.447994
[6] H. W. Kroto, J. R. Heath, R. F. Curl, R. E. Smalley, Nature 1985, 318, 162–163. DOI: 10.1038/318162a0
[7] S. Feldman in www.thirteen.org/bucky/dare.html
[8] H. W. Kroto, Angew. Chem. Int. Ed. 1992, 31, 111–129. DOI: 10.1002/anie.199201113
[9] J. R. Heath, S. C. O’Brien, Q. Zhang, Y. Liu, R. F. Curl, H. W. Kroto, F. K. Tittel, R. E. Smalley, J. Am. Chem. Soc. 1985, 107, 7779–7780. DOI: 10.1021/ja00311a102
[10] W. Krätschmer, K. Fostiropoulos, D. R. Huffmann, Chem. Phys. Lett. 1990, 170, 167. DOI: 10.1016/0009-2614(90)87109-5
[11] R. Taylor, J. P. Hare, A. K. Abdul-Sada, H. W. Kroto, Chem. Commun. 1990, 1423–1425. DOI: 10.1039/C39900001423
[12] C. S. Yannoni, P. P. Bernier, G. Meier, J. R. Salem, J. Am. Chem. Soc. 1991, 113, 3190–3192. DOI: 10.1021/ja00008a068
Author
Professor Helena Dodziuk, Warsaw, Poland
Also of Interest
- Exciting Aspirin,
Helena Dodziuk,
ChemistryViews 2013.
DOI: 10.1002/chemv.201300001
Aspirin was first made in 1897 and is the most widely used medicine in the world, so how does it remain an exciting drug? - Women in Chemistry — Interview with Helena Dodziuk,
Vera Köster,
ChemistryViews 2011.
DOI: 10.1002/chemv.201000114
H. Dodziuk, Polish Academy of Sciences, works on molecules with interesting topography and strained hydrocarbons with unusual spatial structures



I was working as Senior Assistant Editor on Chem Comm when the paper from R. Taylor, J. P. Hare, A. K. Abdul-Sada, H. W. Kroto arrived in our offices…exciting times. We did our best to get it published as quickly as possible (this was pre-web, everything still on actual paper, although we did have email) without breaking the editorial rules. Unfortunately, if I remember rightly, Krätschmer’s Nature paper on C60 beat us into print by a week or so despite our best efforts…