Useful but Toxic – The Mitsunobu Reaction
The Mitsunobu reaction is a classic of organic synthesis. It is used to convert an alcohol into various functional groups, such as esters, on their way from starting materials to final product. It was first published in 1967 by Oyo Mitsunobu, who died 10 years ago. His team used triphenylphosphine and an azodicarboxylate, diethyl azodicarboxylate (DEAD), or diisopropyl azodicarboxylate (DIAD) to induce a condensation reaction between the alcohol and a phosphoric or carboxylic acid.
.gif)
Scheme 1. The classic Mitsunobu reaction.
A key feature of the Mitsunobu reaction is that it proceeds under mild conditions with an inversion of the starting materials’ stereochemistry. Moreover, chemists have extended the reaction way beyond carboxylates to phenols, sulfonamides, azides, thiols, and activated methylenes. But despite the utility of these various flavors of the Mitsunobu reaction, they all produce hydrazines that must be disposed of, and the commonly employed reagent, DEAD, is toxic as well as representing an explosion risk.
Greener and Safer
Daisuke Hirose, Tsuyoshi Taniguchi, and Hiroyuki Ishibashi of Kanazawa University, Japan, have used an iron catalyst and atmospheric oxygen to make the Mitsunobu reaction “greener” and safer.
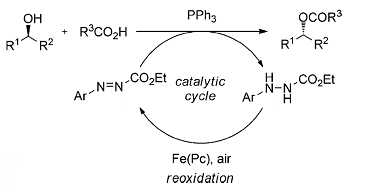
Researchers had previously attempted to improve the reaction by making the DEAD component behave catalytically at lower concentration by using a re-oxidizing agent in the form of iodobenzene diacetate. Taniguchi and colleagues recently demonstrated how alkoxycarbonyl radicals can be generated by using an iron(II) catalyst and induce aerobic oxidation of carbazates. They hoped that this catalyst, which contains a phthalocyanine unit, might be useful in a modified Mitsunobu, acting to re-oxidize DEAD. Unfortunately, the masked nitrogen atoms in the hydrazine waste product that is to be re-oxidized do not succumb to the reagent’s advances.
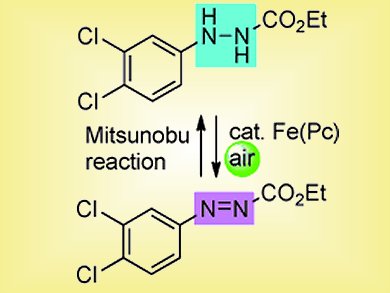
Writing in a recent edition of Angewandte Chemie, the team has now demonstrated that they can carry out a Mitsunobu reaction with ethyl 2-phenylazocarboxylate instead of DEAD and that the waste product from this reaction, ethyl 2-phenylhydrazinecarboxylate, can be re-oxidized by their inexpensive and non-toxic iron(II)- phthalocyanine catalyst because delocalization on the phenyl ring stabilizes the intermediate and facilitates the oxidation on the nitrogen atom. They describe their “delight” in finding this possible route into a recycling Mitsunobu reaction.
Scheme 2. Concept for a catalytic Mitsunobu reaction.
Final Step to a Recyclable Reagent
Unfortunately, this was not the last hurdle. The Mitsunobu reaction required stoichiometric amounts of ethyl 2-phenylazocarboxylate and the re-oxidation was a separate reaction. When the team tested the combined system for catalysis, they obtained no product. Their rationale was not to be defeated, however, and they carried out a systematic screening of different solvents, additives, concentrations, and temperatures until they discovered the optimal conditions. This was followed by modifying the hydrazinecarboxylate. They found that ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate worked, and worked best.
“The Mitsunobu reaction has already proved invaluable in drug discovery,” synthetic chemist Richard Hartley of the University of Glasgow, UK, told ChemViews magazine. “The tremendous advantages of using oxygen as the final oxidant and only catalytic amounts of a hydrazine derivative means it may now be considered for larger-scale industrial processes where chemists have previously been reluctant to employ it.” He sees great potential for the new approach: “This breakthrough and the ongoing optimization of the conditions should revitalise use of the reaction, bringing it up to modern “green” standards. In short, giving a middle-aged reaction the green shoots of youth.”
- Recyclable Mitsunobu Reagents: Catalytic Mitsunobu Reactions with an Iron Catalyst and Atmospheric Oxygen,
Daisuke Hirose, Tsuyoshi Taniguchi, Hiroyuki Ishibashi,
Angew. Chem. Int. Ed. 2013, 52, 4613–4617.
DOI: 10.1002/anie.201300153