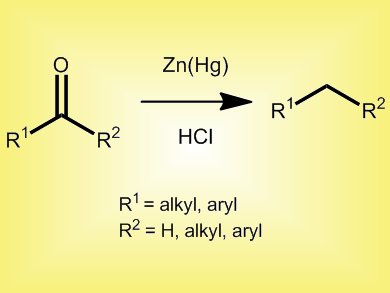
The Clemmensen reduction of ketones and aldehydes is the simplest direct method for converting a carbonyl group into a methylene group. This year marks the 100th anniversary of Erik Christian Clemmensen’s first report of the reduction that now bears his name. His initial investigations centered on the reduction of alkyl-aryl ketones. These ketones were known to be stable to heating with zinc and HCl, but Clemmensen discovered that use of a zinc-mercury amalgam as a catalyst instead of zinc, produced the corresponding hydrocarbon (pictured).
The reduction takes place at the surface of the zinc catalyst, but the mechanism is still poorly understood. Alcohols were initially postulated as intermediates, but subjection of the corresponding alcohols to these same reaction conditions does not lead to alkanes [1]. Recently, it has been suggested that two pathways, involving ionic and nonionic reactions, are involved. In this situation, the reduction is promoted by a single electron transfer from Zn to the ketone or aldehyde.
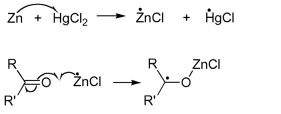
This forms a carbon radical bound to the Zn through the oxygen atom of the carbonyl group. Subsequently, it is thought that a range of different radical species are formed through several single electron transfers [2].
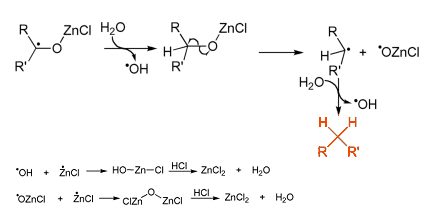
The original conditions reported by Clemmensen required using aqueous HCl heated to reflux, so today milder versions are used. For example, the use of powdered zinc in organic solvents such as ether or tetrahydrofuran. These anhydrous conditions have found great synthetic utility in modern chemistry, such as in the reduction of a tricyclic ketone in the preparation of an anticancer agent (see Scheme 1).
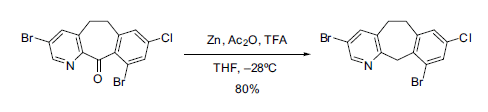
Scheme 1. Reduction of a tricyclic ketone under modified Clemmensen conditions [3].
References
[1] E. Vedejs, Clemmensen Reduction of Ketones in Anhydrous Organic Solvents, in Organic Reactions, John Wiley & Sons, New York, USA, 1975, 22, 401–422.
[2] Z. Wang, Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, New York, USA, 2009. ISBN: 978-0-471-70450-8
[3] S. Caron, Practical Synthetic Organic Chemistry, John Wiley & Sons, New York, USA, 2011. ISBN: 978-1-118-09356-6
Details on Clemmensen’s life from: Dansk Biografisk Leksikon Online, Gyldendal, Copenhagen, Denmark, 2011. Link
Erik Christian Clemmensen is the answer to Guess the Chemist 18, which gave details about Clemmensen’s life.