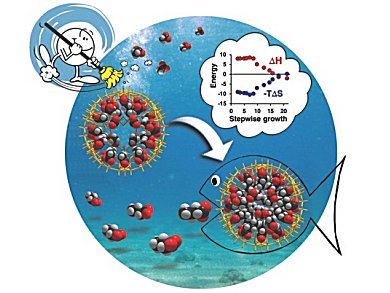
The hydrophobic interaction corresponds to the tendency of hydrophobic groups to cluster together in water. It it is one of the most important driving forces in nature. Hydrophobic effects are responsible for numerous processes including micelle formation, protein folding, and host/guest encapsulation. Despite numerous investigations, very little experimental data is available concerning this multistep process.
Achim Müller, Universität Bielefeld, Germany, and Ira Weinstock and Alina Grego, Ben Gurion University of the Negev, Beer Sheva, Israel, solved this challenging fundamental problem. Their experiment is based on the step-wise growth of a structurally well-defined organic aggregate within a water-soluble porous metal-oxide nanocapsule.
The nanocapsule is a (pentagon)12(linker)30-type anionic complex, [1(acetate)22(H2O)16]34−, where 1 ({MoVI6O21(H2O)6}12{(MoV2O4)30) refers to the inorganic capsule framework.
The weakly bound acetate ions of the [1(acetate)22(H2O)16]34− capsule, and approximately 35 unbound water molecules that are not shown in the formula, are displaced stepwise by the controlled uptake of one to 24 n-butyrate ions. This results in a micelle-like (n-butyrate)24 assembly with a practically water-free central cavity. The porous capsule allows to control the micelle forming process and makes it possible to investigate the underlying energetics associated with each step.
To reveal the thermodynamic signatures of these individual steps, the temperature dependence of the equilibrium constants were determined by 1H NMR spectroscopy.
The authors say, this method promises new access to the understanding of the individual steps of biologically related and other important hydrophobic assembly processes.
- Stepwise-Resolved Thermodynamics of Hydrophobic Self-Assemby: A New Experimental Concept,
Alina Grego, Achim Müller, Ira A. Weinstock,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201303083