Mefloquine (MQ), a known antimalarial drug, is commercially available as a racemate where both enantiomers have been shown to display biological differences. The (+)-MQ is more active on some Plasmodium strains, whereas the (–)-MQ is known to act as an adenosine receptor agonist; such binding is believed to be responsible for neuropsychiatric side effects such as depression and psychosis.
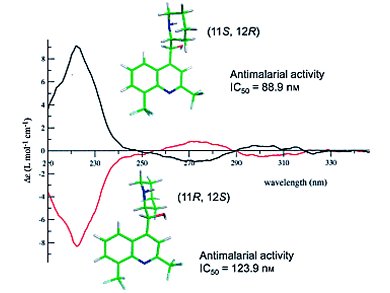
A number of researchers have attempted to determine the absolute configuration of erythro-mefloquine enantiomers in the past. Uwe M. Reinscheid and Christian Griesinger, Max-Planck-Institut für Biophysikalische Chemie, Göttingen, Germany, Birger Dittrich, Georg-August-Universität Göttingen, Germany, and co-workers have determined the X-ray crystal structures two mefloquine enantiomeric Mosher amides. Meanwhile, Pascal Sonnet, Université de Picardie Jules Verne, Amiens cedex, France, and colleagues reported the X-ray crystallography, CD spectroscopy, and molecular modelling of both MQ enantiomers along with their respective antimalarial activities.
In these two publications, the absolute configuration of (+)-erythro-mefloquine has been determined as (11S, 12R).
- Absolute Configuration and Antimalarial Activity of erythro-Mefloquine Enantiomers,
Alexandra Dassonville-Klimpt, Christine Cézard, Catherine Mullié, Patrice Agnamey, Alexia Jonet, Sophie Da Nascimento, Mathieu Marchivie, Jean Guillon, Pascal Sonnet,
ChemPlusChem 2013.
DOI: 10.1002/cplu.201300074 - The Absolute Configuration of (+)- and (−)-erythro-Mefloquine,
Michael Müller, Claudia M. Orben, Nina Schützenmeister, Manuel Schmidt, Andrei Leonov, Uwe M. Reinscheid, Birger Dittrich, Christian Griesinger,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201300258