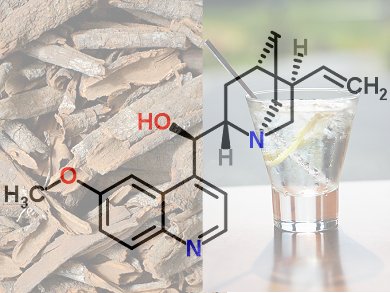
Cinchona, or quina, refers to a genus of about 38 species in the family Rubiaceae, first found in forests of the tropical Andes. Known to natives in Peru as “quinaquina” (bark of the barks), it is perhaps the greatest gift from the New World to the Old World. In particular, its bark yielded the first effective remedy for malaria, one of the most dangerous of infectious diseases. The chief component of cinchona bark, quinine, is still used as a medication, but also for preparing tonic water and Bitter Lemon.
There is thus good reason for having a closer look from a chemical point of view at this bitter tree bark. … And the long road from the structure determination to the total synthesis of quinine is an exciting detective story.
7. From Molecular Formula to a Total Synthesis
7.1 First Delusions and Confusions
Due to strong demand for quinine, coupled with a chronic shortage of the substance with respect to the European colonial powers, August Wilhelm von Hofmann, German Director of the Royal College of Chemistry in London, UK, encouraged around 1850 an attempt at synthesizing the substance. At that time neither the tetrahedral theory of carbon nor the ring structure for benzene was known, and one had no notion of the three-dimensional makeup of molecules. Hofmann’s synthetic plans were thus limited of necessity to counting the various atoms in the starting materials, and balancing them against those in the products.
On the basis of a preliminary molecular formula—C20H22N2O2, determined exclusively on the basis of combustion analyses and later shown to be wrong—he suggested a transformation of “naphthalidine” (today known as α-naphthylamine) into quinine [11]:
2 C10H9N + 2 H2O → C20H22N2O2
naphthylamine + water → quinine
As to how such a hydration was to be carried out in practice, Hofmann speculated rather boldly: “Of course we cannot anticipate causing water to react through a simple contact, but a fortunate experiment might achieve this goal through discovery of an appropriate metamorphic process.”
After Adolph Strecker in 1854 corrected the quinine molecular formula to C20H24N2O2 [12, 13], one of Hofmann’s pupils, William Perkin, who at the time was only 18 years old, seized upon the idea once again and, in accord with the new molecular formula, selected a starting material with four additional hydrogen atoms, namely N-allyltoluidine. His plan was to oxidize the amine with potassium dichromate, to give quinine according to the following equation:
2 C10H13N + 3 O → C20H24N2O2 + H2O
N-allyltoluidine + oxygen → quinine + water
Perkin attempted to carry out the transformation in his own very modest private laboratory, whereby he acquired—instead of the hoped for white quinine—an insoluble, noncrystallizable, red-brown precipitate. But Perkin did not give up. He proposed getting to the bottom of this transformation, so he decided to repeat the oxidation reaction with the simpler amine aniline, which was readily available from coal tar. It must have been clear to him that quinine itself could not be produced from aniline, but presumably he hoped that from reaction of the simpler starting material he could gain insight into the course of the process.
The reaction product—a black, amorphous residue—at first glance looked useless. But Perkin was persistent, and with the aid of alcohol he was able to isolate from the black mass a deep violet substance. With this pigment he carried out preliminary dye experiments, using one of his sister’s white silk blouses, and later, in 1868, he patented his violet dye, which eventually acquired the name “mauve”.
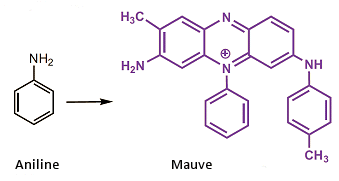
Perkin himself ultimately determined that his mauve must be a mixture of at least two compounds, and that the “aniline” he had started with contained rather large amounts of o– and p-toluidine, so that these structural elements must of necessity have found their place in the reaction products. The structural formula present for decades in the literature for mauve proved in 1994 to be wrong. That reproduced above is the correct formula for the principal component of Perkin’s mauve. [14, 15]
This rather audacious chemical approach—as seen from today’s perspective—led to the first of the aniline dyes, and the chance discovery marked the beginning of the almost unbelievable ascent of the coal tar (or aniline) dye industry
But that was only the beginning! It later turned out that not only textiles, but also microorganisms (Gram staining test for bacteria) and subcellular components (chromosomes, where chroma is Greek for color) can also be dyed. The next step was application of dye derivatives as chemotherapeutics. The major German corporations Farbwerke Hoechst AG (Hoechst Dye Works Inc.), Agfa (Aktiengesellschaft für Anilinproduktion = Corporation for Aniline Production), and BASF (Badische Anilin und Soda Fabrik = Baden Aniline and Soda Factory) point to this origin in their very names.
7.2 Structure Determination for Quinine
Determining the complete structures for quinine and other cinchona components kept generations of chemists busy. From the many small pieces, the German chemist Paul Rabe was able to solve the structural puzzle in 1908, and propose the ultimately confirmed constitutional formula for quinine (Fig. 4, 1) [16]. In this process, quinine was disassembled, with full retention of configuration—in other words very carefully, and observing all the rules of the art—until one finally had in hand exclusively smaller molecules, the configurations of which were already known.
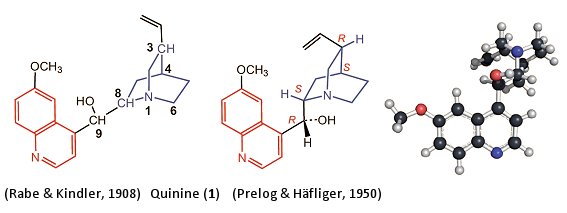
Figure 4. Structure of quinine.
Quinine turned out to be a methoxy-substituted quinoline ring (Fig. 4, red), bound by way of a carbon atom to a bicyclic quinuclidine (blue) [17–19]. Even the nitrogen atom N1 is stereogenic, in that its free electron pair, directed outwards, can be regarded formally as a fourth substituent. Due to the rigidity of the bicyclic quinuclidine ring system, the configurations of the two bridgeheads N1 and C4 must be identical, hence, there is no need to make explicit reference to the S configuration of N1.
The story of establishing the configuration, and a great deal else about quinine as well, and has been presented in wonderfully readable form by Fritz Eiden [17, 18].
It was not until 1950 through the work of Prelog and Häfliger [19], and their establishment of the configuration of C9, that the structure of quinine could be definitively regarded as fully determined (Fig. 4) [20–22]. The relative configurations, previously established strictly by chemical means, were confirmed by X-ray structural analysis in 1955.
7.3 The Long Journey to the First Total Synthesis of Quinine
Strictly speaking, a total synthesis of a natural product should commence with a set of elements drawn from the periodic table. In practice, however, one does not start with elemental carbon, oxygen, hydrogen, and nitrogen, but takes advantage instead of the tremendous reservoir of compounds already prepared by generations of chemists. The goal then becomes preparation using the fewest possible steps, starting from simpler, readily available—perhaps commercially available—previously synthesized components.
The total synthesis of quinine evolved over the course of nearly a century, and reflects increasing knowledge about the chemistry of quinine, along with developments in synthetic methodology.
.gif)
Figure 5. The long road to a total synthesis of quinine.
1853: It all started with Louis Pasteur, who, by warming quinine in dilute acid, rearranged it to the isomeric quinotoxine (2), though he had no idea about the actual chemical structures of either one (Fig. 5) [23].
1918: Paul Rabe succeeded in establishing the correct molecular formula for quinine, and together with Karl Kindler converted quinotoxine (2) back into quinine (1) via a three-step reaction sequence (Fig. 5) [23].
1943: M. Proštenik and V. Prelog dismantled quinonine (4)—another component of cinchona bark, one which is closely related to quinine—into (+)-homomeroquinene (3) [25], which together with quinic acid (5) they then converted into (+)-quinotoxine (2) (Fig. 5).
A coupling of this sort with quinic acid had been described earlier by Rabe and Kindler in their synthesis of the analogous “dihydro-quinotoxine” [26]. Thus, a quinotoxine partial synthesis was accomplished from quinonine, another natural product. The authors also pointed the way toward a total synthesis:
“Since … the transformation of quinotoxine into … quinine … was accomplished some time ago by Rabe and Kindler, all that remains for a total synthesis of quinine is solution of the problem of synthetic preparation of homomeroquinene.”
1944: Precisely this goal was achieved by R. B. Woodward and W. E. Doering in the form of a brilliant synthesis. Starting with 7-hydroxyisoquinoline (6) they first synthesized racemic homomeroquinene (3) [27, 28], which they converted with quinic acid by the method of Proštenik and Prelog into racemic quinotoxine (2). With the aid of a D-tartaric acid derivative, (2) was separated into its two enantiomers. Since Rabe and Kindler had in 1918 already converted (+)-quinotoxine into quinine (1), Woodward and Doering entitled their publication “The Total Synthesis of Quinine”. Today one would speak more precisely of a formal total synthesis, since the authors themselves had not actually carried out the last three steps.
Scientifically, the Total Synthesis of Quinine was a Bombshell
In only slightly more than a year, these two 26-year-old American chemists had, in about 20 synthetic steps, prepared one of the most sought-after of all natural products (Fig. 6).
.gif)
Figure 6. The Woodward and Doering (formal) total synthesis of quinine.
The outstanding creativity of Woodward’s synthetic planning was frequently referred to in the chemical professional literature [29]. One thing stood out immediately at even a superficial glance: in the first half of the synthesis one would never guess where the whole thing was headed. Why, for example, did Woodward and Doering introduce the methyl group shown in red in Fig. 6 into the isoquinoline ring?
This step turns out to represent an especially difficult synthetic task, since the reaction of 7 to 8 resisted proceeding correctly. Only after 16 hours of heating with sodium methanolate at 220 °C in an autoclave (!) was it finally accomplished, in 65 % yield. It is a miracle that under these drastic conditions anything at all remained. Only at the end does it become apparent why so much effort was expended here: this methyl group was to become a part of the requisite vinyl group.
Paul Rabe, who first gained access to this publication only after the end of the Second World War, handwrote in 1948 a moving letter to Woodward, reproduced in [30]. The partial translation that follows (also from [30]) is by O. T. Benfey and R. Huisgen: “I studied your first paper with admiration. I am delighted that I have lived to see the total synthesis of quinine, and I send you my sincere congratulations. Your thought was certainly a fruitful one to increase the four carbon atoms of the benzene nucleus of isoquinoline by an additional one and then with the help of these five carbon atoms to create the rest of the propionic acid and the vinyl group!”
Still today, synthetic chemists speak of this synthesis with high regard [31]. “It was Woodward’s first total synthesis, was admired, was at the time an important and unprecedented achievement, and remained a scientific milestone. The Woodward–Doering synthesis of quinine was also groundbreaking for organic synthesis in the decades that followed … This achievement made him in his field almost a demigod.”
The total synthesis of quinine in 1944 was not just any synthesis, however; in the middle of the Second World War it was also an important wartime achievement from a strategic point of view. A large fraction of the American force was deployed in Southeast Asia, and their wellbeing in malaria-infested regions was dependent upon this drug. The major cinchona bark exporting lands, like Java, at that time a Dutch colony, as well as the sea passages, were all controlled by the Japanese enemy, so the United States suffered from a chronic shortage of this medication so vital for the troops. One can only imagine the elation on the home front when a successful laboratory synthesis was finally achieved, something totally independent of enemy supply sources. The American media raised Woodward and Doering overnight to superstars. The New York Times exulted in May 1944 [32]: “The war has brought forth many a wonder of research but none more wonderful than the success of Drs. Robert B. Woodward and William E. Doering in synthesizing quinine. Behind this outstanding achievement stands nearly a century of vain effort—vain partly because organic chemistry had not developed the conceptions and techniques required. Yet the failures contributed much to ultimate success by indicating the path to be pursued.”
The journalists couldn’t get it through their heads that with this (formal) total synthesis, quinine itself had not been produced at all. This minor scientific detail was ignored: for the American nation, in the middle of the Second World War, Woodward and Doering were simply heroes. Whether Woodward and Doering defended themselves energetically enough against this false impression, or whether they—along with chemists in general—simply savored the fame that had broken out, remains an open question. In any case, never again has a research achievement in synthetic organic chemistry found such a positive resonance among the populace as in 1944 the “total synthesis” of quinine.
The commotion in the press at the time provides us with unusual insight into the way Woodward and Doering operated, which is not so apparent from close examination of their original publication. Thus, the latter makes no mention of Doering’s dogged battle with the devil that sat in the preparative details. In numerous subsequent interviews, journalists coaxed especially Doering to talk about their difficulties and emotions. One example will illustrate the point: in the original publication, the sensitivity of (9) was mentioned only in passing, and in sober journal language [33]: “It was necessary to handle the amino-esters (9) with care, since heat brought about changes which rendered the material useless for further synthetic work, probably through inter- and intramolecular condensations involving the amino and the carbethoxyl groups”.
The aminoester 9 apparently decomposes if warmed, with elimination of ethanol, so that the condensation products were deficient by one oxygen atom. In an interview with the distinguished magazine “The New Yorker”, Doering in 1944 described what this decomposition unleashed in him emotionally [33]: “It took twelve weeks for (this) step because we kept losing a key oxygen atom. Lord knows where it went, but it wasn’t there. You have no idea how depressed we got … By September … we had found the atom, [and] each move was taking only five weeks …”
Doering’s account of the last days of the quinine synthesis is also moving. By Easter of 1944, the synthesis of racemic quinotoxine had already been successful, and the racemate had been resolved. It was only necessary still for natural (+)-quinotoxine to be crystallized and unequivocally identified. Doering described this moment [33]: “From nine-thirty in the mornings until four-thirty the following mornings we mixed and stirred and heated. All we got was a dirty, brown oil. We dropped in a few natural crystals [of quinotoxine] because sometimes they act like seeds and make other crystals, but nothing happened. April tenth we got synthetic crystals, all right, but they acted badly—wouldn’t purify completely—so we had an ice-cream soda and we went home at three A.M. It was Woodward’s birthday and I’ve never been so blue.” Next morning the crystals did purify … .
“At eleven o‘clock I went into the darkroom for the final test, a complicated matter of measuring the rotation of the crystals” [33].
[What this relates to, of course, is measurement of optical rotations, established using a polarimeter. Rotation of a crystal is not involved, but rather the plane of linearly polarized light.]
“It came out absolutely correct! I ran back to the lab and said, ‘Woodward, this is it!’ Woodward went in and took a look and came out smiling. We shook hands … I get sick thinking of the details, but we worked fourteen months—February first, 1943, to April eleventh, 1944, at eleven A.M. Boy, what a moment!”
7.4 Gilbert Stork’s Footnote Number 14
With constant improvements in synthetic methods, new total or partial syntheses were attempted, ones that promised to either be shorter or give higher yields [31]. One high point was the first stereocontrolled total synthesis of quinine, by Gilbert Stork (Fig. 7) [34].
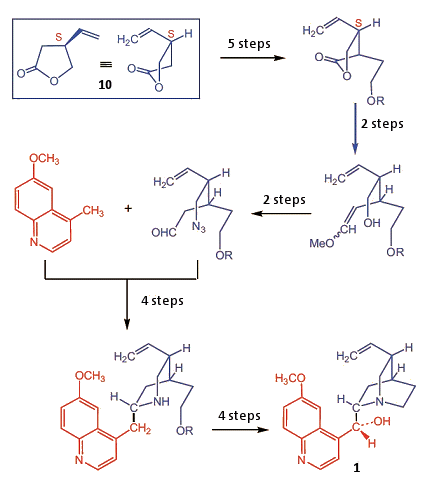
Figure 7. Stereocontrolled total synthesis by Stork, et al. (2001).
The synthesis began with the chiral component (10), the configuration of which must remain unchanged throughout the multistep synthetic sequence. That alone represents a significant synthetic challenge, since it means that only selective reactions can be utilized. Stork’s success was due to his use of a surprising, innovative synthetic strategy consisting of a scant 20 reaction steps, elegantly selected.
Stork’s stereocontrolled total synthesis of quinine was highly praised as a master stroke [35]. Even more than with respect to its scientific value, a stir was created by a particular footnote, one that raised serious questions regarding the “total” synthesis of quinine by Woodward and Doering 50 years earlier:
“Woodward and Doering did not claim to have confirmed Rabe’s 1918 report, in a few lines, that he had succeeded in converting quinotoxine to quinine (although the basis of their characterization of Rabe’s claim as “established” is unclear), nor is there any evidence that they produced any quinine in their own laboratories. But this was wartime, and the U.S. had been cut off from the Dutch East Indies, its major source of cinchona bark. The resulting anxiety may explain press accounts, notable for enthusiasm rather than for sober analysis, which created the quasiuniversal impression that the construction of homomeroquinene in 1944 meant that quinine had been synthesized.”
It must be emphatically acknowledged that Gilbert Stork never expressed the slightest doubt regarding the brilliance of their total synthesis of homomeroquinene. He wrote in a letter to the editor of Chemical and Engineering News in 2001 [36]: “… [The synthesis] is beautiful and inspiring … and Doering’s superb and insufficiently acknowledged mastery of the far from trivial experimental difficulties is what makes the homomeroquinene synthesis a masterpiece”.
Rabe and Kindler’s Work Not Reproducible?
Stork’s criticism applied above all to the final paragraph of Woodward and Doering’s publication: “In view of the established conversion of quinotoxine to quinine, [24] with the synthesis of quinotoxine the total synthesis is complete”. In fact it was the adjective “established” that Stork was unwilling to accept. In his opinion, the transformation of quinotoxine into quinine was far from “established”, in that Rabe and Kindler in their short communication [24] had not provided any experimental details, and even later had not done so. In laying claim to a total synthesis, Woodward and Doering should necessarily have verified experimentally Rabe and Kindler’s synthetic steps.
For Stork, footnote 14 was the best part of his work [37]. Many saw things differently, however, and found Stork’s attack on the deceased Bob Woodward (1917–1979, Nobel Prize for Chemistry 1965) at the least to be very strange, while others regarded it as a form of insult.
What might have led the otherwise so prudent, humorous, and universally highly esteemed Stork [38] to such an attack?
Perhaps it was a disappointment from long ago, when Woodward, in 1944, failed to reply to a letter from the young student Stork. In this letter, Stork asked for what seemed to him an important detail [40]: “… Would you also tell me whether Rabe’s conversion of quinotoxine into quinine has been repeated by you in your recent work … “
Be that as it may, the Woodward–Doering total synthesis was for Stork a myth: “This was an impressive achievement. But it wasn’t quinine” [37]. This and many other of his utterances sparked a very emotional discussion about whether Rabe and Kindler’s publication was at all reliable, whether Woodward and Doering had been too naive, or if they perhaps were in fact aware—or at least suspected—that Rabe and Kindler’s work might be nonreproducible, or if Stork simply out of wounded vanity over disregard of his earlier request really wanted to chip away at Woodward’s almost superhuman image.
The editors of Chemical & Engineering News, the weekly news magazine of the American Chemical Society, dashed on ahead, in that the editor-in-chief spoke of a historic “setting the record straight” [39]. On the other side, there were many voices indicating that Woodward and Doering could quite rightly build upon the integrity and preparative skill of Rabe and Kindler [40].
Since the passionate discussion regarding Woodward and Doering’s quinine synthesis has died down somewhat, thanks to temporal distance, we can in the meantime analyze the facts as these are now understood, and come to our own conclusions.
7.5 Who Can Trust Whom, and in Whom Can We Place Our Trust?
In the controversial communication “Über die partielle Synthese des Chinins” (On the Partial Synthesis of Quinine), Rabe and Kindler reported their transformation of quinotoxine into quinine (Fig. 8). Rabe had already presented in detail in 1911 the preparative execution of the first two steps in an analogous transformation of quinotoxine into cinchoninone [41]. In the third step they introduced a new reduction method involving aluminum powder in sodium ethanolate/ethanol, which in their judgment represented, “a significant advance in syntheses in the cinchona alkaloid series.”
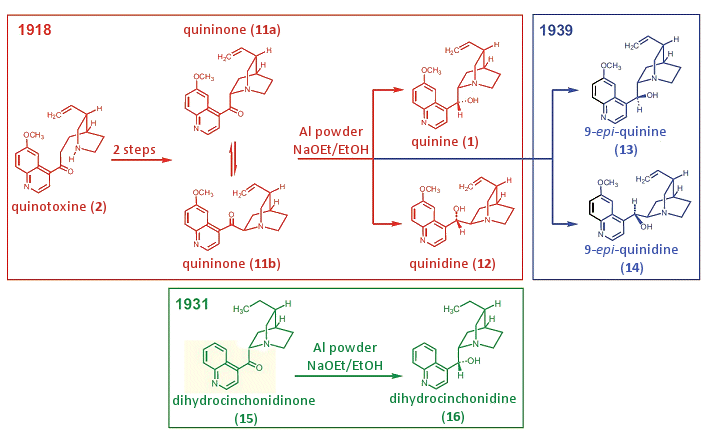
Figure 8. The Rabe–Kindler partial synthesis of quinine from quinotoxine.
The actual experimental procedure is extremely tersely described, however: “16.3 g of previously synthesized quininone (today: quinidinone) produced, upon treatment with the stated reduction mixture [aluminum powder in sodium ethanolate/ethanol], alongside 0.9 g of quinidine, the quinine in a yield of 2 g of analytically pure substance.”
In alkaline solution, quininone (11a) is in equilibrium with the isomeric quinidinone (11b). Since quinidinone is less soluble, it precipitates first. Thus, Rabe did not isolate quininone, but rather quinidinone. This is actually irrelevant for the subsequent reduction, however, since in alkaline medium an equilibrium between the two isomers is again established. In the structural formulas of Fig. 8 we indicate this equilibrium between the isomers.
Melting points, optical rotations, and elemental analyses were reported for both of the isolated reaction products, and these were in agreement with data for authentic samples.
If one did not propose to accuse Rabe and Kindler of perpetrating an awkward fraud, then there could be no doubt that these reactions were actually carried out as described. Nevertheless, it was customary then as now that, in a short communication in which one was declaring priority for a successful synthesis, it was expected, within a specific period of time, generally one to two years, that this would be followed by a complete presentation that included preparative procedures. Why Rabe and Kindler failed to conform in this case is unknown. Or should it perhaps not in fact be interpreted as a failure on their part? We need to examine this matter a bit more closely.
In 1932, Rabe once again took up the reduction method involving aluminum powder, this time in a work entitled “Über die Reduktion der China-Ketona zu China-Alkoholen” (Regarding the Reduction of the Cinchona Ketones to Cinchona Alcohols) [42]: “Non-catalytic hydrogenation is achieved with the aid of aluminum powder and sodium ethylate in alcoholic solution. This method, introduced by P. Rabe and K. Kindler [24], has not yet been described thoroughly in the literature. For this reason it will be illustrated using the example of dihydrocinchonidinone (15)” (Fig. 8).
Although the procedure that followed was detailed, Stork pointed out correctly that it was not strictly the reduction of quininone to quinine, but “only” that of the analogous dihydrocinchonidinone (15), containing in place of a vinyl group the much less sensitive ethyl group (Fig. 8).
Stork addressed directly the structural difference in question [37]: “Among other things, that compound does not have the vinyl group that’s present in quinotoxine. Whether the recipe would work for a substrate containing such a reactive group is not clear”.
This objection is enlightening, since with any reduction involving hydrogen one runs the risk that the vinyl group will be hydrogenated. Rabe was very well aware of this, however, and pointed out at the very beginning of the experimental section the fundamental difference between “catalytically activated” hydrogen (at a Pt-catalyst) and “nascent hydrogen” (H2 formation at aluminum powder in NaOEt/EtOH). There follows the crucial preparative notation: “The ketones were reduced partly with catalytically activated, partly with nascent hydrogen. The first method is applicable only with ketones that are free of vinyl groups, and the second, and this is important, also with vinyl-containing ketones.”
Thus, Rabe noted specifically that a reduction mixture consisting of aluminum powder and sodium ethanolate in ethanol does not (!) attack double bonds. Presumably, Rabe believed that with this general procedure, as annotated, he had also described thoroughly enough the reduction of quininone to quinine.
This presumption was confirmed by Rabe and Kindler in their 1939 article “Zu Synthesen in der Reihe der China-Alkaloide” (Regarding Syntheses in the Cinchinone Series) [43]. They had developed new procedures for separating epi-quinine (13) and epi-quinidine (14) from complex mixtures, and “… this circumstance put us in a position to terminate the study initiated in 1918. At that time we were successful, thanks namely to discovery of a new non-catalytic method of hydrogenation, in a partial synthesis of the pair quinine and quinidine, starting thereby with quinotoxine, which still lacked a closed quinuclidine ring”.
They then took up once again the over 200 g residue from the year 1918. In actual fact they isolated this time, in addition to quinine (1) and quinidine (12), the non-natural stereoisomers (13) and (14) from the old reaction mixture.
Also in this article they once more extolled the definitive advantage of the “non-catalytic” reduction method involving aluminum powder in sodium ethanolate/ethanol: “We used for hydrogenation namely aluminum powder in alcoholic solution in the presence of sodium ethoxide, a reducing agent that can indeed attack the carbonyl group, but not—or only with sufficient difficulty—another unsaturated group, the vinyl group, which is readily reduced by catalytically activated hydrogen.”
It is thus unequivocally the case that Rabe and Kindler actually did carry out the partial synthesis of quinine from quinotoxine. Publication of the experimental details for the last three steps was simply delayed, for reasons which remain unknown. This publication behavior was unusual for Rabe, but one cannot somehow take that to be a sign of untrustworthiness. Overall one also cannot accuse Woodward and Doering of having had so much doubt regarding the work of Rabe and Kindler that they needed to repeat these steps. Doering commented in 2007 with respect to this: “We relied on the work of one of Germany’s best organic chemists to complete the conversion. There was no earthly reason to doubt the validity of Rabe’s work.” [44].
7.6 Paul Rabe and Karl Kindler—Rest in Peace
Finally, the years-long heated debate regarding the quinine total synthesis motivated the chemistry historian Jeff Seeman to review the background in a comprehensive and meticulous way, and then present us with his insights in an amusing-to-read fashion [30].
Last but not least, in 2008 the Rabe–Kindler reaction sequence from quinotoxine to quinine was reconstructed in terms of the laboratory techniques of 1944, by Smith and Williams [45]. The result came as no surprise: in a rough-and-ready fashion, quinine can indeed be prepared from quinotoxine by the Rabe–Kindler method. The biggest problem was that modern aluminum powder turned out to be unsuitable because it was too “fresh”, and had to be aged for a week or two in air. The results appeared in Angewandte Chemie under the unusually melodramatic title “Rabe Rest in Peace: Confirmation of the Rabe–Kindler Conversion of d-Quinotoxine to Quinine. Experimental Affirmation of the Woodward–Doering Formal Total Synthesis of Quinine”.
This publication, which was supposed to—once and for all—put an end to the tempest in a teapot, was addressed by Stork as follows: “I think Williams and Smith did an excellent job of figuring out how to modify aluminum powder so they could reproduce the Rabe–Kindler portion of the Woodward–Doering route to quinine.”
Too bad, though, that Stork couldn’t refrain from stoking the fire one more time [46]: “It’s a shame that this wasn’t done by Woodward and Doering.”
Complexity of the Chemical Research Process
A review of the quinine total syntheses shows that research results are the product not only of supposedly objective laboratory work, but also of people with colorful characters, working in a socio-political setting at a particular point in time. Thus, the quinine synthesis can serve as an epistemological lesson in the complexity of the process of chemical research [47].
From a less aloof point of view we might simply enjoy the recognition that chemists—even the great ones—don’t always think and deal as strictly rationally as they often believe they must purport, but instead do so emotionally and full of passion. This is in fact what actually makes them into endearing people.
References
[11] W. H. Perkin, J. Chem. Soc. 1896, 69, 596–637. DOI: 10.1039/CT8966900596
[12] Z. H. Skraup, Ber. Dtsch. Chem. Ges. 1878, 31, 1516–1519. DOI: 10.1002/cber.18780110265
[13] Z. H. Skraup, Liebigs Ann.Chem. 1879, 199, 344–359. DOI: 10.1002/jlac.18791990209
[14] O. Meth-Cohn, M. Smith, J. Chem. Soc., Perkin Trans. 1, 1994, 5. DOI: 10.1039/p19940000005
[15] M. M. Sousa et al., Chem. Eur. J. 2008, 14, 8507. DOI: 10.1002/chem.200800718
[16] P. Rabe, Ber. Dtsch. Chem. Ges. 1908, 41, 62. DOI: 10.1002/cber.19080410118
[17] F. Eiden, Pharm. Unserer Zeit, 1998, 27, 257. DOI: 10.1002/pauz.19980270606
[18] F. Eiden, Pharm. Unserer Zeit, 1999, 28, 11 and 74. DOI: 10.1002/pauz.19990280107 and DOI: 10.1002/pauz.19990280208
[19] V. Prelog and O. Häfliger, Helv .Chim. Acta 1950, 33, 2021. DOI: 10.1002/hlca.19500330708
[20] H. Mendel, Proc. K. Ned. Akad. Wet. 1955, 58, 132.
[21] P. M. Kimpenda and L. V. Meervelt, Acta Cryst. 2010, E66, 2443. DOI: 10.1107/S1600536810034288
[22] The absolute configuration was determined in 1967: O. L. Carter et al., J. Chem. Soc. A, 1967, 365. DOI: 10.1039/j19670000365
[23] L. Pasteur, C. R. Hebd. Seances Acad. Sci. 1853, 37, 110. Link
[24] P. Rabe and K. Kindler, Ber. Dtsch. Chem. Ges. 1918, 51, 466. DOI: 10.1002/cber.19180510153
[25] M. Proštenik and V. Prelog, Helv. Chim. Acta, 1943, 26, 1965. DOI: 10.1002/hlca.19430260622
[26] P. Rabe, K. Kindler, Ber. Dtsch. Chem. Ges. 1919, 52, 1842. DOI: 10.1002/cber.19190520908
[27] R. B. Woodward, W. E. Doering, J. Am. Chem. Soc. 1944, 66, 849. DOI: 10.1021/ja01233a516
[28] R. B. Woodward, W. E. Doering, J. Am. Chem. Soc. 1945, 67, 860. DOI: 10.1021/ja01221a051
[29] Classics in Total Synthesis II, K.C. Nicolaou and S. A. Snyder, Wiley-VCH, Weinheim, Germany, 2003.
[30] J. I. Seeman, Angew. Chem. Int. Ed. 2007, 46, 1378. DOI: 10.1002/anie.200601551
[31] T. S. Kaufman, E. A. Rúveda, Angew. Chem. Int. Ed. 2005, 44, 854. DOI: 10.1002/anie.200400663
[32] New York Times, 1944, May 5, 18.
[33] C. Orr, P. Hamburger, The New Yorker, 1944, May 13, 19.
[34] G. Stork et al., J. Am. Chem. Soc. 2001, 123, 3239. DOI: 10.1021/ja004325r
[35] Only one of the many enthusiastic appraisals: S. M. Weinreb, Nature 2001, 411, 429. DOI: 10.1038/35078178
[36] G. Stork, Chem. Eng. News 2001, Oct. 22, 8. Link
[37] A. M. Rouhi, Chem. Eng. News 2001, May 7, 54. Link
[38] J. I. Seeman, Angew. Chem. Int. Ed. 2012, 51, 3012. DOI: 10.1002/anie.201200033 See also http://cenblog.org/newscript/2012/03/gilbert-stork-on-how-not-to-dispose-of-a-steak/
[39] M. Jacobs, Chem. Eng. News 2001, May 7, 5. Link
[40] For example, in Quinine, Definately…, Paul Docherty, Totally Synthetic, 2008, Feb. 6. Link
[41] P. Rabe, Ber. Dtsch. Chem. Ges. 1911, 44, 2088. DOI: 10.1002/cber.19110440318
[42] P. Rabe, Justus Liebigs Ann. Chem. 1932, 492, 242. DOI: 10.1002/jlac.19324920112
[43] P. Rabe, K. Kindler, Ber. Dtsch. Chem. Ges. 1939, 72, 263. DOI: 10.1002/cber.19390720206
[44] B. Halford, Chem. Eng. News 2007, Feb. 26, 47. Link
[45] A. C. Smith, R. M. Williams, Angew. Chem. Int. Ed. 2008, 47, 1760. DOI: 10.1002/anie.200705421
[46] B. Halford, Chem. Eng. News 2008, Feb. 4, 8. Link
[47] K. A. F. D. Souza, P. A. Porto, J. Chem. Educ. 2012, 89, 58. DOI: 10.1021/ed1003542
Prof. Klaus Roth
Freie Universität Berlin, Germany.
Dr. Sabine Streller
Freie Universität Berlin, Germany.
The article has been published in German in:
and was translated by W. E. Russey.
From Pharmacy to the Pub — A Bark Conquers the World: Part 1
The quinine-containing bark of the Cinchona tree is probably the most valuable drug the Americas gave the world.
From Pharmacy to the Pub — A Bark Conquers the World: Part 2
Thomas Buchler, CEO of the quinine producer Buchler & Co., talks about the ups and downs of the international cinchona trade.
From Pharmacy to the Pub — A Bark Conquers the World: Part 4
Last but not least, besides explaining how quinine helps against malaria, we talk about drinks containing this wonderful alkaloid, no matter whether stirred or shaken.
See all articles by Klaus Roth published by ChemViews magazine