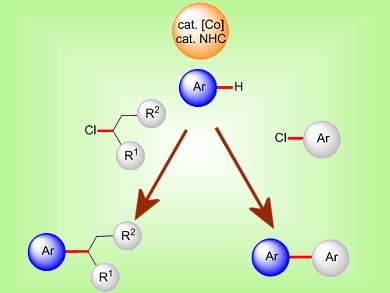
Regioselectively substituted biaryls are key structural motifs in bioactive compounds, natural products, and advanced functional materials. This makes catalyzed, direct arylations of arenes an important tool in organic synthesis. Up until now these reactions have mostly been achieved with relatively expensive second-row transition-metal complexes. Protocols that utilize naturally more abundant first-row transition-metal catalysts for these reactions remain scarce.
Lutz Ackerman and co-workers, Institut für Organische und Biomolekulare Chemie, Göttingen, Germany, have reported the development of inexpensive cobalt catalysts derived from [Co(acac)2] (acac=acetylacetonate) and N-heterocyclic carbene (NHC) precursors for direct H bond arylations and alkylations of heteroaryl-substituted arenes and heteroarenes with cost-effective chlorides as electrophiles. The C–H bond functionalizations proceeded with low catalyst loadings at ambient temperature and with excellent chemo- and site-selectivities.
Detailed studies on the scope of cobalt-catalyzed direct alkylations with secondary alkyl halides are currently ongoing in this group.
- Cobalt-Catalyzed C–H Bond Functionalizations with Aryl and Alkyl Chlorides,
Benudhar Punji, Weifeng Song, Grigory A. Shevchenko, Lutz Ackermann,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201301409