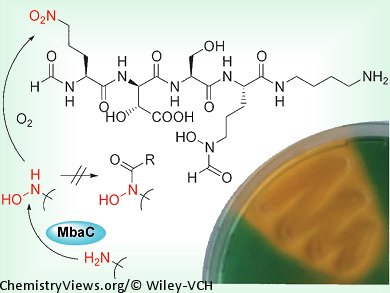
A team of scientists based in Germany have elucidated the structure and absolute configuration of malleobactin, which is the siderophore of the pathogenic Burkholderia mallei. Siderophores are strong iron-binding agents, and act as virulence factors for pathogenic bacteria.
Christian Hertweck and his co-workers, Friedrich-Schiller-Universität Jena, Germany, investigated the siderophores of B. mallei and B. pseudomallei, which can lead to often lethal infections. Gene cluster analyses and targeted knock-outs were used to correlate the siderophore activity to the mba gene cluster in B. thailandensis. Structural analysis of a pure sample of malleobactin A showed that one of the component amino acids contains a terminal nitro group. It was shown to be 2-amino-5-nitropentanoic acid, which has not been reported as being part of a natural product to date. The team then examined the biosynthesis of malleobactin, and showed that it originates from an unprotected hydroxylamine group, which is oxidized to a nitroso derivative and subsequently to the observed nitro compound. In other related compounds, the hydroxylamine group is protected by acylation to form a hydroxamate, which not only prevents overoxidation, but also acts as a chelator.
As well as elucidating the structure of the virulence factor malleobactin, this study has also identified an unprecedented amino acid in a natural product and a new route toward aliphatic nitro compounds.
- Nitro versus Hydroxamate in Siderophores of Pathogenic Bacteria: Effect of Missing Hydroxylamine Protection in Malleobactin Biosynthesis,
Jakob Franke, Keishi Ishida, Mie Ishida-Ito, Christian Hertweck,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201303196