Low-Carbon, Hydrogen Economy
Carbon is big business chemically speaking, from diamonds to the whole of the petrochemicals industry, from the lead in your pencil to the enormous nanoscopic potential of the latest wonder material grapheme. Somewhere in the last decade or so another form of carbon rolled off the laboratory bench – the fullerenes. But, as the notion of a low-carbon, hydrogen economy is mooted for our future energy needs it seems that functionalized fullerene salts might become a critical part of that new world.
Writing in the journal Angewandte Chemie, in a VIP paper, researchers from Peking University, Beijing, and the Chinese Academy of Science (CAS), China, have demonstrated how salts of octa-hydroxy-[60]fullerene, a modified form of the archetypal, buckyball – more succinctly known as a fullerenol –, can markedly catalyze the electrochemical evolution of hydrogen gas from water.
Hydrogen-Evolution Reactions
Junqiao Zhuo, Tanyuan Wang, Lu Liu, Liangbing Gan, and Meixian Li at Peking and Gang Zhang of the CAS, explain that as carbon-based fuel sources dwindle or are eventually pushed to the margins in the era of sustainable power, the ability to split water to generate hydrogen gas, an alternative fuel, will become increasingly important. The precious metals, platinum, palladium, and ruthenium have been the focus of much research into the catalyzed hydrogen-evolution reactions (HERs). But these elements are rare, expensive, and of limited or otherwise restricted supply, even if recycling is taken into consideration. Less costly metals, such as nickel and cobalt, have been tested, but they suffer from corrosion problems and markedly lower catalytic activity. If only there were a carbon-based alternative that could be obtained sustainably and at much lower cost than any precious metal …
Fullerenol Catalysts
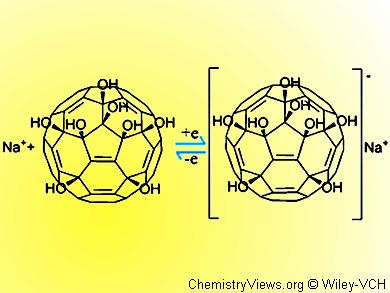
Li and colleagues have turned to the polyhydroxylated fullerenes (fullerenols) as one possible solution. These compounds have been widely studied, can be water soluble, have low toxicity, and can scavenge free radicals. However, the synthesis of specific fullerenols usually results in complex mixtures, which has stymied their development somewhat. The team recently described a novel and specific synthesis for making a C60 with eight OH groups.
The team carried out initial tests with an electrode containing this compound in its coating using cyclic voltammetry to measure the response to an applied voltage. The results are promising. The activity could be improved and optimal conditions approached by using an aqueous sodium or potassium chloride solution rather than pure water.
The team suggests that the alkali metal ions are readily intercalated into the fullerenol crystal lattice and so counteract the negative charge and stabilize the coating.
The team demonstrated a three-step reduction for their fullerenol electrode with a Nafion layer with hydrogen evolution. This finding compared favorably to the same test set up using unmodified [60]fullerene. The electrochemical reaction mechanism, thus revealed, suggests that a one-electron-transfer reduction and reoxidation takes place with sodium salt depositing and then being released from the electrode in these two steps. Exchange current density was on a par with that observed for well-developed molybdenum and tungsten sulfide catalysts, thus boding well for the use of fullerenol electrodes in HER.
“We anticipate our discovery to be the starting point for exploring more effective all-carbon-based non-metal molecular catalysts for the HER,” the team concludes. This new work pushes research into hydrogen evolution catalysts away from expensive noble metals and towards a more sustainable and less costly area of chemistry in the form of carbon compounds.
Currently the team is working on other fullerene derivatives with different polar functional groups, such as sulfo and carboxyl groups as well as different numbers of hydroxy groups, to optimize the catalytic activity in HER.
- Salts of C60(OH)8 Electrodeposited onto a Glassy Carbon Electrode: Surprising Catalytic Performance in the Hydrogen Evolution Reaction,
Junqiao Zhuo, Tanyuan Wang, Gang Zhang, Lu Liu, Liangbing Gan, Meixian Li,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201305328