The condensation reaction of carboxylic acid and alcohol is known as esterification. This is one of the most fundamental transformations in organic chemistry, but an asymmetric version was not produced until recently, as reported by the team of Kenya Nakata, Shimane University, and Isamu Shiina, Tokyo University of Science, both Japan.
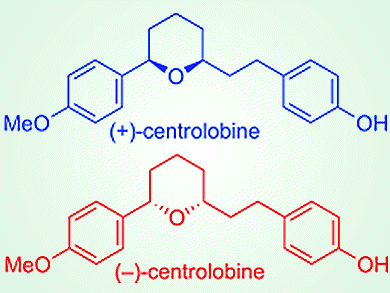
Kinetic resolution of racemic alcohols with achiral carboxylic acids in the presence of chiral nucleophilic organocatalysis by asymmetric esterification is a suitable method for the direct production of chiral carboxylic esters. To demonstrate the utility of the asymmetric esterification, the team applied it to the enantiodivergent synthesis of both enantiomers of centrolobine, which is an anti-inflammatory compound. Both (+)- and (–)-centrolobine (pictured) were successfully synthesized in a total of seven steps.
This new synthetic strategy will hopefully be applicable to the synthesis of other biologically active chiral compounds.
- An Enantiodivergent Synthesis of (+)- and (−)-Centrolobines by Asymmetric Esterification Catalyzed by (R)-(+)-N-Methylbenzoguanidine ((R)-NMBG),
Kenya Nakata, Tatsuya Tokumaru, Hidetoshi Iwamoto, Yutaka Nishigaichi, Isamu Shiina,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300139