Nanoparticles are often used in analytical sciences and biomedicine. Colloidal nanomaterials are of particular interest as redox tags for electrochemical biosensing devices because they have unique properties, such as stable fluorophore components, which are tunable and can enable optical and spectrometric encoding of target molecules. Furthermore, such nanomaterials often exhibit electrochemical activity, with a diverse range of redox potentials, that can be used to provide voltammetric signatures in bioassays.
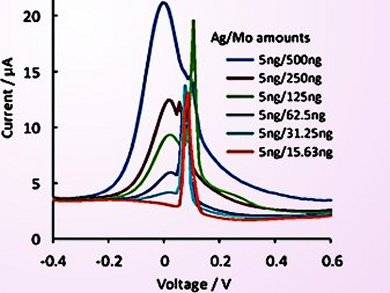
Tatsuro Goda, Nanyang Technological University, Singapore, and Martin Pumera, Medical and Dental University (TMDU), Chiyoda, Tokyo, Japan, together with their co-workers investigated the oxidative response of both Ag and Mo nanoparticles (+84 and 57 mV, respectively) by using differential pulse voltammetry. After identifying the individual responses, the two materials were combined on a single electrode for simultaneous detection. Even when the Ag and Mo nanoparticles coexisted on the same electrode, the individual oxidative signals could be resolved at both high and low Ag/Mo ratios.
The electrochemical features of Ag and Mo nanoparticles were demonstrated to be compatible with encoding techniques and can, therefore, be used as redox tags in multiplex sensing regimes for the detection of target analytes in point-of-care devices.
 Simultaneous Electrochemical Detection of Silver and Molybdenum Nanoparticles,
Simultaneous Electrochemical Detection of Silver and Molybdenum Nanoparticles,
Tatsuro Goda, Adriano Ambrosi, Yuji Miyahara, Martin Pumera,
ChemElectroChem 2014.
DOI: 10.1002/celc.201300225
ChemPubSoc Europe’s new journal ChemElectroChem is dedicated to covering the entire scope of pure and applied electrochemistry.