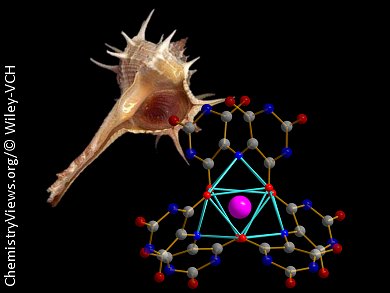
Tyrian purple, an optical dye first used by the Phoenicians in 1500 BC, is secreted by sea snails of the Muricidae group. The molecule responsible for the deep-purple color is 6,6′-dibromoindigo. The synthetic optimization of purple dyes has led to a wide family of derivatives from the Murex family, possessing a similar skeleton. From this family, murexide, the ammonium salt of 2,6-dioxo-5-[(2,4,6-trioxo-5-hexahydropyrimidinylidene)amino]-3H-pyrimidin-4-olate, has been used for decades as a complexometry indicator in water or in non-aqueous solvents. In the presence of metal ions, even in very dilute solution, a color change occurs from deep purple to orange. Although many complexes of transition metals with this ligand have been reported, Kevin Bernot, Université Européenne de Bretagne, France, with his group and collaborators from Université de Rennes, Université Lyon, and Université Claude Bernard Lyon, all France, have only now structurally characterized the first lanthanide complexes of murexide.
They obtained single crystals of lanthanide–murexide adducts for Ln = Sm–Lu, and Y, which have a 1:3 metal-to-ligand stoichiometry and are discrete complexes. This is significantly different from what had been previously postulated. The complexes showed single-ion magnet behavior, which could be correlated with their NIR emission properties.
These complexes are potential lanthanide-based molecular precursors because they present external amine and carbonyl groups able to further coordinate metal ions, which in turn may give rise to extended homo- or heteropolynuclear magnetic and NIR emissive architectures.
- Unraveling the Crystal Structure of Lanthanide–Murexide Complexes: Use of an Ancient Complexometry Indicator as a Near Infrared-Emitting Single-Ion Magnet,
X. Yi, K. Bernot, V. Le Corre, G. Calvez, F. Pointillart, O. Cador, B. Le Guennic, J. Jung, O. Maury, V. Placide, Y. Guyot, T. Roisnel, C. Daiguebonne, O. Guillou,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201303833