Gold-based catalysts have intrigued chemists because of their favorable properties for selective oxidation reactions. One type of Au-based catalyst that has attracted significant attention is nanoporous Au, because it can dissociate atomic oxygen and resists deactivation. Nanoporous Au is typically prepared by removing Ag from a Ag/Au alloy until the structure becomes very porous and only a small amount of Ag remains. This residual Ag has been shown to play a key role in oxygen activation during catalytic reactions.
In order to better understand the mechanisms behind these processes, Cynthia M. Friend, Harvard University, Cambridge, USA, and colleagues have investigated how thin films of Ag/Au alloys with different compositions interact with molecular oxygen and their use in the low temperature oxidative coupling of methanol. With this model system, they found that the alloy composition influences the temperature at which atomic oxygen recombines. In addition, the presence of mixed Ag/Au sites gives these alloys more favorable properties than either pure Ag or pure Au.
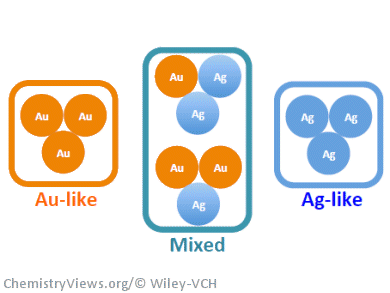
These experimental results are supported by a statistical model correlating the percentages of Au-like, Ag-like, and mixed Ag/Au sites present in these alloys and the amount of methyl formate formed.
- Ag/Au Mixed Sites Promote Oxidative Coupling of Methanol on the Alloy Surface,
Bingjun Xu, Cassandra G. F. Siler, Robert J. Madix, Cynthia M. Friend,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304837