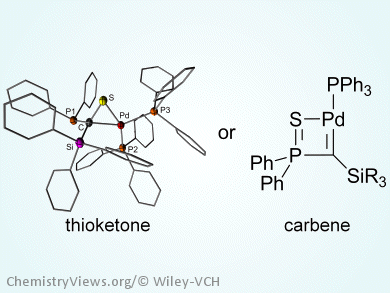
Carbene complexes have become essential to many organic transformations. Depending on the substituents at the carbenic carbon atom and the involved reactivity they are often classified into Fischer- and Schrock-type complexes. Geminal dianions represent precursors for the preparation of a new class of carbene complexes, which are distinguishable from the Fisher- and Schrock-type complexes by their unique electronic structure in which the metal carbon bond is formally formed by a four-electron donation from the ligand to the metal. Alternatively, these complexes can be accessed via stabilized Li/Cl carbenoids.
Viktoria Gessner and co-workers, Julius-Maximilians-Universität Würzburg, Germany, report the preparation of palladium thioketone and T-shaped carbene complexes by treatment of thiophosphoryl substituted Li/Cl carbenoids with a Pd0 precursor. Depending on the steric demand, the anion-stabilizing ability of the silyl moiety, by negative hyperconjugation effects, and the remaining negative charge at the carbenic carbon atom, isolation of a three-coordinate, T-shaped palladium carbene complex is possible. In contrast, insufficient charge stabilization results in the transfer of the sulfur of the thiophosphoryl moiety and thus in the formation of a thioketone complex.
.jpg)
Whereas the thioketones are stable compounds the carbene complexes are revealed to be highly reactive and decompose under elimination of Pd metal.
- Substitution Effects on the Formation of T-Shaped Palladium Carbene and Thioketone Complexes from Li/Cl Carbenoids,
Sebastian Molitor, Kai-Stephan Feichtner, Claudia Kupper, Viktoria H. Gessner,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304927
A contribution to the upcoming European Young Chemist Special Issue to be featured in Chemistry – A European Journal.