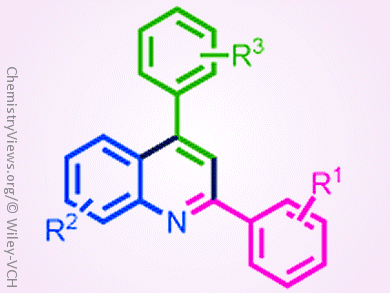
The synthesis of quinolines and their derivatives has attracted great attention owing to their potential application in organic materials and medicinal chemistry. However, syntheses that are cost effective, environmental friendly, and have simple operating methods are still rare.
Chien-Hong Cheng and co-workers, National Tsing Hua University, Hsinchu, Taiwan, developed a convenient iron-catalyzed coupling reaction for the synthesis of 2,4-disubstituted quinolines from simple starting materials, that is, aldehydes, amines, and styrenes. The features of this reaction include broad substrate scope, inexpensive FeCl3 catalyst, oxygen as the oxidant, and no hazardous byproducts. Primary mechanistic studies suggest that the reaction might be an electrophilic aromatic substitution (SEAr) type cyclization.
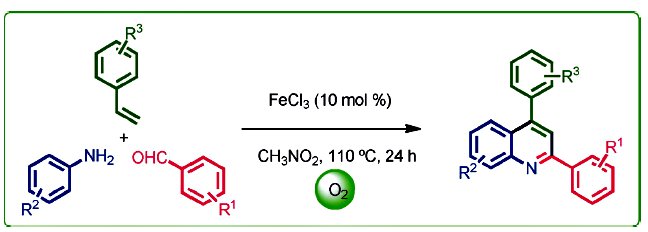
The new method provides an easy and environmental friendly way for the synthesis of substituted quinolines that might increase the possibility of exploring the applications of quinolines.
- Synthesis of Substituted Quinolines by Iron(III)-Catalyzed Three-Component Coupling Reaction of Aldehydes, Amines, and Styrenes,
Parthasarathy Gandeepan, P. Rajamalli, Chien-Hong Cheng,
Asian J. Org. Chem. 2014, 3, 303–308.
DOI: 10.1002/ajoc.201300262