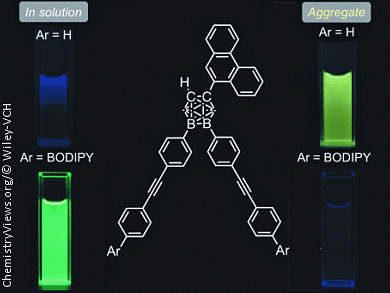
By synthesizing a series of o-carborane-containing compounds that contain a combination of three different π-conjugated units, phenanthrene, diphenylacetylene (tolane), and boron-dipyrromethene (BODIPY), Yasuhiro Morisaki and co-workers, Kyoto University, Japan, have demonstrated a method for controlling the emission properties of organic dyes, which are important in organic electronics, through functional groups and physical states.
Generally, when a π-conjugated substituent is at the C1 (and/or C2) position of o-carborane aggregation-induced emission (AIE; emission in the aggregated state) occurs, but emission in solution does not occur. On the other hand, common emission behaviors are observed when a π-conjugated substituent is at the B9 (and/or B12) position, i.e., the compounds do not emit in the aggregated state due to aggregation-caused quenching (ACQ) but do emit in solution. Thus, the emission behaviors and colors of C1,B9,B12-trisubstituted-o-carborane compounds can be precisely controlled by their physical state and the π-conjugated substituents on the o-carborane core.
The group hopes that it will be possible to produce an organic white-light-emitting material with a single o-carborane-based molecule.
- Control of the Emission Behaviors of Trifunctional o-Carborane Dyes,
Masato Tominaga, Hirofumi Naito, Yasuhiro Morisaki, Yoshiki Chujo,
Asian J. Org. Chem. 2014, 3, 624–631.
DOI: 10.1002/ajoc.201300280