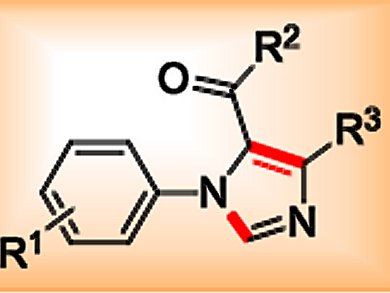
1,5-Disubstituted imidazole derivatives are of great interest to both medicinal and synthetic chemists because of their unique structure and biological profile. As such, considerable research efforts have been devoted to the development of efficient strategies for their synthesis. Recently, visible-light-induced photoredox catalysis has enjoyed a remarkable renaissance and found wide applications in the synthesis of various biologically important carbocycles and heterocycles.
The group of Jia-Rong Chen and Wen-Jing Xiao, Central China Normal University, Wuhan, China, reported a cascade reaction between secondary amines and isocyanides to yield 1,5-disubstituted imidazoles. The reaction begins with visible-light-induced photocatalytic aerobic oxidation of the amine using an iridium catalyst with phenylpyridine and bipyridine ligands. Subsequent [3+2] cycloaddition of the resulting imine with the isocyanide reaction partner is followed by aromatization, which yields the desired imidazole product.
The reaction shows wide substrate scope as well as high functional-group tolerance and provides the corresponding 1,5-disubstituted imidazoles in generally good yields. The readily availability of the starting materials, simple operation, and mild reaction conditions make this method attractive to both organic and medicinal chemists.
- De Novo Synthesis of Imidazoles by Visible-Light-Induced Photocatalytic Aerobic Oxidation/[3+2] Cycloaddition/Aromatization Cascade,
Qiao-Hui Deng, You-Quan Zou, Liang-Qiu Lu, Zi-Long Tang, Jia-Rong Chen, Wen-Jing Xiao,
Chem. Asian J. 2014.
DOI: 10.1002/asia.201402443