The primary focus over the past 30 years of research into aldol methodology has been on the development of catalytic, enantioselective methods for selectively obtaining single stereoisomers. Largely these challenges have been met by very inspired and elegant solutions and the aldol reaction has provided a useful testing ground for the development of modern asymmetric catalysis. Despite these successes, a remaining obstacle in aldol methodology is the synthesis of quaternary stereogenic centers.
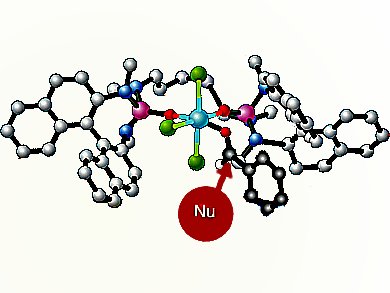
Scott E. Denmark and co-workers, University of Illinois, Champaign, IL, USA, have addressed this problem by developing silyl ketene imines, derived from a variety of α-branched nitriles, as reagents for the construction of quaternary stereogenic centers by means of the aldol addition reaction. In the presence of SiCl4 and the catalytic action of a chiral phosphoramide, silyl ketene imines undergo extremely rapid and high-yielding addition to provide a wide variety of aromatic aldehydes with excellent diastereo- and enantioselectivity.
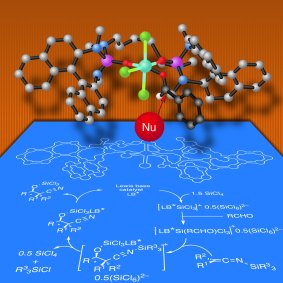
Of particular note are the high yields and selectivities obtained from electron-rich, electron-poor, and hindered aldehydes. Semiempirical calculations conducted by the group provided a rationalization of the observed diastereo- and enantioselectivity via open transitions states.
- Enantioselective Construction of Quaternary Stereogenic Carbon Atoms by the Lewis Base Catalyzed Additions of Silyl Ketene Imines to Aldehydes,
Scott E. Denmark, Tyler W. Wilson, Matthew T. Burk,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201403342