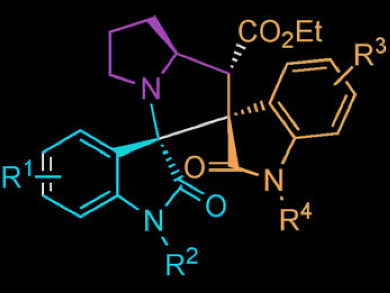
An efficient synthesis of spirooxindole derivatives is highly valued due to the pronounced biological activities of this class of compounds.
Min Shi and colleagues, East China University of Science and Technology / Chinese Academy of Science, Shanghai, have developed a highly regio- and stereoselective synthesis of bispirooxindoles. In the three-component reaction, azomethine ylides are generated in situ from isatin derivatives and proline, which can then react with different electron-deficient alkenes in a 1,3-dipolar cycloaddition, finally affording bispiro scaffold compounds in excellent yields.
The stereochemistry was determined by single-crystal X-ray analysis.
 Highly Efficient and Stereoselective Construction of Bispirooxindole Derivatives via a Three-Component 1,3-Dipolar Cycloaddition Reaction
Highly Efficient and Stereoselective Construction of Bispirooxindole Derivatives via a Three-Component 1,3-Dipolar Cycloaddition Reaction
Dr. Qin Xu, De Wang, Yin Wei, Min Shi
ChemistryOpen 2014.
DOI: 10.1002/open.201402003