Separation of Para and Ortho Water
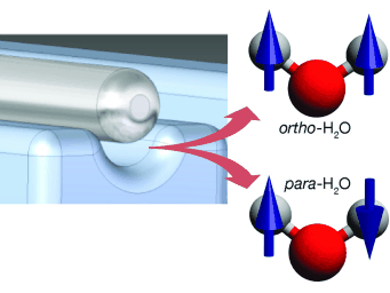
Not all water is equal—at least not at the molecular level. There are two versions of the water molecule, para and ortho water, in which the spin states of the hydrogen nuclei are different. In the journal Angewandte Chemie, German researchers have now reported the successful separation of the two forms.
Spin is a quantum mechanical value that can be visualized as the intrinsic angular momentum of a particle rotating around its own axis. A hydrogen nucleus (proton) can adopt two different states, comparable to rotation clockwise and counterclockwise. In the case of water, the nuclear spins of the two—indistinguishable—protons can be combined in four different ways: one antisymmetric and three symmetric wavefunctions. Water adopting the antisymmetric wavefunction is called para water, whereas water adopting one of the symmetric ones is called ortho water. Because switching from one state to the other is “forbidden” due to quantum-mechanical symmetry rules, the two spin isomers cannot interconvert without external influences such as collisions.
Freezing and Isolating Spin States
A team at the Center for Free Electron Laser Science (DESY and University of Hamburg) led by Jochen Küpper and Daniel Horke has now successfully separated the two spin isomers and prepared isolated, highly pure samples of para and ortho water.
The challenge was to “freeze” the spin states by diluting single water molecules to such an extent that they could no longer collide with each other. To achieve this, the researchers use a noble gas high-pressure chamber charged with one droplet of water. When the chamber’s pulse valve is opened, the mixture shoots into a vacuum chamber at supersonic speeds, cooling rapidly due to the extremely fast expansion. This results in a focused beam of very cold water molecules that are so far from each other that they cannot induce a spin flip in each other.
For the separation, the researchers made use of the fact that para and ortho water molecules do not have the same quantum states. In a strong inhomogeneous electric field, the accelerated water molecules are deflected from their flight path by a different amount depending on their quantum state.
Applications of Spin-Pure Water
Possible applications for spin-pure water extend to many fields. Astrophysicists have determined that the ratio of ortho water to para water in interstellar ice is different from what is expected. Spin-pure water could enable revealing laboratory experiments. Techniques such as nuclear magnetic resonance spectroscopy (NMR) could benefit as their sensitivity could be increased by the use of para water in the hydration shells of proteins, improving the determination of their structure.
- Separating Para and Ortho Water,
Daniel A. Horke, Yuan-Pin Chang, Karol Długołęcki, Jochen Küpper,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201405986