Interview with Hideki Yorimitsu
My favorite drink 10 years ago was … beer with my students.
And today … beer with my family.
My greatest motivation 10 years ago was … unveiling epoch-making organic reactions.
And today … achieving my dream that my students and postdocs as well as myself will continue to enjoy chemistry forever.
My most exciting discovery in the past 10 years has been … Pummerer annulation, which has made a contribution to the recent renaissance of organosulfur chemistry.
The most significant scientific advance of the past 10 years has been … the emergence of the science of atomic layers as well as the discovery of induced pluripotent stem cells.
I’m glad that … the range of chemistry that I can cover … has changed in the past 10 years.
| Hideki Yorimitsu
|
|
|
Date of birth |
January 22, 1975 |
|
Position |
Associate Professor, Kyoto University, Japan |
|
Homepage |
|
|
Education |
B.S. 1997, Kyoto University Ph.D. 2002, Kyoto University Postdoc 2002-2003, University of Tokyo |
Featured Article
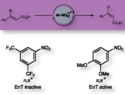
- Activator-Free Palladium-Catalyzed Silylation of Aryl Chlorides with Silylsilatranes,
Yutaro Yamamoto, Hiroshi Matsubara, Kei Murakami, Hideki Yorimitsu, Atsuhiro Osuka,
Chem. Asian J. 2014.
DOI: 10.1002/asia.201402595Palladium-catalyzed silylation of aryl chlorides has been found to take place with a new class of disilane, silylsilatranes, under activator-free conditions. Experimental and computational studies have revealed that smooth silyl transfer from silylsilatranes to arylpalladium chloride is facilitated by strong and specific interaction between the Lewis acidic silatrane unit and the chloride. This paper contains important information about as yet unexplained transmetalation to realize activator-free cross-coupling reactions.
- See all interviews in ChemViews Magazine