Active pharmaceutical ingredients (APIs), such as ibuprofen, are classed as essential medicines by the World Health Organization (WHO). Given the demand for APIs, continuous-flow synthesis is becoming an appealing option for their production.
David R. Snead and Timothy F. Jamison, Massachusetts Institute of Technology (MIT), Cambridge, USA, have developed a continuous-flow synthesis of ibuprofen that has distinct advantages over a previously reported procedure, namely that the synthesis avoids the use of triflic acid and PhI(OAc)2. The new synthesis comprises three chemical transformations that take place within three minutes and use commonly available reagents and minimal solvents. Furthermore, toxic and aggressive reagents can be delivered safely and the exothermic reactions and quenching of AlCl3 proceed without problems.
The first step involves a solvent-free Friedel-Crafts acylation between isobutylbenzene and propionyl chloride, where the AlCl3 was delivered neat in the latter reagent. Quenching the acylation reaction with 1M HCl did not result in reactor clogging. The next step involves 1,2-aryl migration with trimethyl orthoformate and neat ICl (iodine monochloride). DMF was added to dissolve the solid iodine that was formed. After quenching of the ICl with 2-mercaptoethanol, the ester was hydrolyzed to form ibuprofen sodium carboxylate.
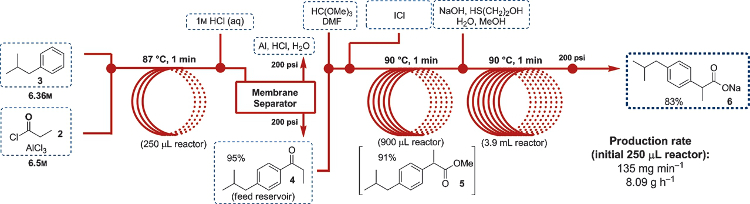
The researchers were able to scale up their synthesis so that it formed 8.09 grams of product per hour, with an overall yield of 83 %. This work shows that large amounts of important APIs can be efficiently generated in reactors that are about half the size of a standard fume hood.
- A Three-Minute Synthesis and Purification of Ibuprofen: Pushing the Limits of Continuous-Flow Processing,
David R. Snead, Timothy F. Jamison,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201409093




