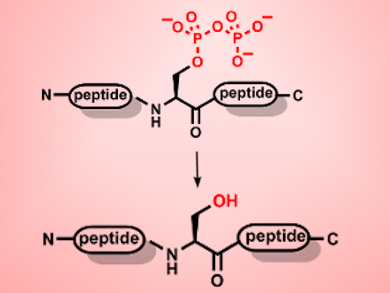
Post-translational modification (PTM) is one of the main ways cells control protein function and regulate their effects. Pyrophosphorylation of proteins is one recently identified PTM. However, its role in cellular events is still largely unknown. There has been some debate about whether this modification is reversible, either chemically or enzymatically, but no de-pyrophosphorylating enzymes have thus far been identified.
Lisa M. Yates, Dorothea Fiedler, Princeton University, New Jersey, USA, and colleagues have synthesized a series of short peptides containing a defined pyrophosphate modification to systematically study pyrophosphorylation in vitro and in cell extracts. They were able to provide the first evidence of the reversibility of pyrophosphorylation. In addition, they found that the pyrophosphate modification is relatively stable to acidic conditions but undergoes hydrolysis in the presence of sulfonic acids. Testing seven known phosphatase enzymes revealed that three of them are capable of removing the pyrophosphate group.
The group also discovered that there are endogenous enzymes that can remove pyrophosphate groups in both yeast and human HeLa cell lysates. This activity was partially reduced by the addition of phosphatase inhibitors, but not completely eliminated. This suggests that there are yet unidentified enzymes responsible for removing the pyrophosphate group in vivo. These peptides should serve as a valuable tool for the continued study of pyrophosphorylation and its role in biochemical signaling.
- Establishing the Stability and Reversibility of Protein Pyrophosphorylation with Synthetic Peptides,
L.M. Yates, D. Fiedler,
ChemBioChem 2015.
DOI: 10.1002/cbic.201402589


But we think that these PTM are used during the traffic of proteins from RE where they are synthesized to their cell target