Challenges for Zeolites
Zeolites are aluminosilicate materials with crystalline framework structures. They are very versatile materials that are widely used by chemists both in the lab and in industry. However, their narrow micropores impose important diffusion limitations on large reactant and product molecules approaching or leaving the active sites. This leads to lowered conversion or undesirable secondary reactions which prompt coke deposition and catalyst deactivation.
Many efforts have been devoted to the synthesis of molecular sieves with larger pore sizes, especially in the mesoscale (2 nm to 50 nm), with the aim to overcome these diffusion problems. In the 90s, a new family of ordered mesoporous silica and aluminosilicate materials, M41S, was developed by the Mobil Oil Company using surfactants to produce tunable mesoporosity. Nevertheless, the amorphous frameworks and thin pore walls of this sort of material result in poorer hydrothermal stability and weaker acidity than that of zeolites. This has inhibited their industrial applications.
Surfactant-Templated Mesostructured Zeolites
It has been a long-standing goal of researchers to develop zeolites with controlled mesoporosity, i.e. mesopore walls composed of a crystalline zeolitic framework. This has been recently achieved using surfactants – like the ones used in the synthesis of amorphous M41S – for the production of surfactant-templated mesostructured zeolites. These materials represent the bridge that closes the gap between microporous crystalline zeolites and mesoporous amorphous materials. They are strongly acidic and highly hydrothermally stable while accessible to very large molecules, making them ideal catalysts for the transformation of bulky molecules.
The presence of controllable intercrystalline mesoporosity in mesostructured zeolites has been confirmed by producing a series of materials with surfactants of increasing length (quaternary ammonium surfactants with aliphatic chains ranging from C10–C22). The structure of the mesoporous zeolites can be clearly seen in transmission electron microscope (TEM) images.
|
|
|
Figure 1. Transmission electron microscopy (TEM) images of (a) conventional and (b) mesostructured Y zeolites, c) density functional theory (NLDFT) pore size distribution curves calculated from Ar (87 K) isotherms, and (d) the tomogram of the mesostructured Y-zeolite crystal prepared using C16 surfactants. |
The TEM image of a conventional Y zeolite crystal shows its rows of lattice reflection lines resulting from the regular atomic structure within the crystal (Fig. 1a, a few are highlighted in red for clarity). The mesostructured zeolite crystal similarly shows crystal lattice lines, but in addition, one can see the larger, lighter colored spots corresponding to the mesopores (Fig. 1b, a few pores are circled in red). The diameter of the generated mesopores nicely correlates with the length of the surfactant molecule (Fig. 1c).
This surfactant-templating technique chemically burrows a network of surface-accessible mesopores into the crystal without materially affecting its crystal structure. By combining rotation electron diffraction and electron tomography, it was possible to directly observe the presence of mesoporosity inside the zeolite crystals. They are homogeneously distributed within the zeolite and highly interconnected (Fig. 1d).
Catalytic Opportunities: The Impact of Enhanced Diffusion in Zeolites on Petroleum Refiners
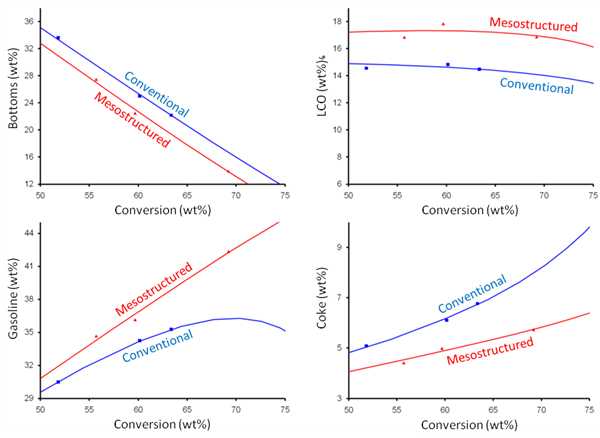
Over the last four decades, modern fluid catalytic cracking (FCC) catalysts have used Y zeolite as the primary active ingredient in the cracking catalysts. The zeolite is a molecular sieve that contains its active sites mainly inside the micropores of the individual crystallites. The size of these pores is typically smaller than that of the molecules that are being converted (e.g., vacuum gas oils), so molecular access to all of the interior catalytic sites is limited by diffusion. These diffusional resistances can impact product selectivity as well as catalyst activity. If the primary cracked fragments are delayed in leaving the zeolite crystal, then they have a higher probability of re-cracking to lighter products. This re-cracking reduces the yield of intermediate desirable products such as light cycle oil (LCO) and gasoline and increases the yield of less valuable, lighter gases, together with coke.
Mesostructured zeolites produced via surfactant-templating have been tested at refinery scale confirming their outstanding catalytic performance and hydrothermal stability. Rive Technology, Monmouth Junction, NJ, USA, has developed methods to reduce the diffusional resistances and increase the yield of the more valuable products. This is achieved by introducing a uniform series of holes into the zeolite crystals themselves, prior to or after incorporation into the finished catalyst.
The surfactant-templated mesopores are significantly larger than the 7.5 Å-diameter micropores which exist naturally in Y zeolite. They have a diameter of about 4 nm if cetyltrimethylammonium bromide (CTAB) is used as a surfactant. The mesopores provide both improved access for large feed molecules to the interior of the zeolite crystal (where the active sites are located), and improved escape of distillate molecules from the zeolite before they re-crack to light gases and coke. As shown in Fig. 2, these unique intercrystalline mesopores are hydrothermally stable in the FCC unit (i.e., they are relatively stable at high temperatures up to about 1,400 °F) and result in a materially improved product yield structure and increased middle distillate yield in catalytic cracking.
|
|
|
Figure 2. Mesoporous ultra stable zeolite Y (USY) shows greatly improved VGO cracking uplifts compared to conventional USY. |
Next Challenges and Opportunities
In less than a decade, surfactant-templated zeolites have gone from the lab to the market place and today, they are a commercial reality. The next challenge for this emerging class of advanced materials is their application in other fields such as biofuel production, gas separation and water treatment, where they are showing outstanding preliminary results. They represent important opportunities both in and beyond catalysis.
The community working on mesoporous zeolites, both in academia and in industry, will gather this summer at the third edition of the International Symposium on Mesoporous Zeolites in Boston, USA, on August 19th, where the first book devoted to this new class of hierarchical materials (Mesoporous Zeolites: Preparation, Characterization and Applications) will be presented .
As Professor Mark Davis says in the foreword of the book: “The synthesis of mesoporous materials can be accomplished via numerous techniques. The amazing number of materials that have emerged from these methodologies illustrate the creativity of the inventors in their designs to simultaneously control composition, structure and textual properties. There is no doubt that much greater understanding of these structure-property relationships will lead to new concepts for applications.”
Selected Publications
- Mesostructured zeolites: bridging the gap between zeolites and MCM-41,
Teerawit Prasomsri, Wenqian Jiao, Steve Z. Weng, Javier Garcia Martinez,
Chem. Commun. 2015, 51, 8900–8911.
DOI: 10.1039/C4CC10391B - Mesoporous Zeolites: Preparation, Characterization and Applications,
E. Li, J. Garcia Martinez (eds.),
Wiley-VCH, Weinheim, 2015.
ISBN: 978-3-527-33574-9 - Realizing the Commercial Potential of Hierarchical Zeolites: New Opportunities in Catalytic Cracking,
Kunhao Li, Julia Valla, Javier Garcia-Martinez,
ChemCatChem 2014, 6, 46–66.
DOI: 10.1002/cctc.201300345 - Evidence of Intracrystalline Mesostructured Porosity in Zeolites by Advanced Gas Sorption, Electron Tomography and Rotation Electron Diffraction,
Javier Garcia-Martinez, Changhong Xiao, Katie A. Cychosz, Kunhao Li, Wei Wan, Xiaodong Zou, Matthias Thommes,
ChemCatChem 2014, 6, 3110–3115.
DOI: 10.1002/cctc.201402499 - Mesoporous materials for clean energy technologies,
Noemi Linares, Ana M. Silvestre-Albero, Elena Serrano, Joaquín Silvestre-Albero, Javier García-Martínez,
Chem. Soc. Rev. 2014, 43, 7681–7717.
DOI: 10.1039/C3CS60435G - A mesostructured Y zeolite as a superior FCC catalyst – from lab to refinery,
Javier García-Martínez, Kunhao Li, Gautham Krishnaiah,
Chem. Commun. 2012, 48, 11841.
DOI: 10.1039/C2CC35659G - Mesostructured zeolite Y – high hydrothermal stability and superior FCC catalytic performance,
Javier García-Martínez, Marvin Johnson, Julia Valla, Kunhao Li, Jackie Y. Ying,
Catal. Sci. Technol. 2012, 2, 987.
DOI: 10.1039/C2CY00309K
Also of Interest
- Characterization of Molecular Highways in Zeolites,
Veronika Belusa,
ChemViews Mag. 2014.
Characterization techniques verify that surfactant-templated zeolites contain intracrystalline and interconnected mesopores - Hierarchical Zeolites Make It to Refinery,
ChemistryViews.org 2012.
First successful commercial scale manufacture of mesostructured zeolite Y promising as fluid catalytic cracking (FCC) catalyst - Improve Zeolites for Catalysis,
ChemistryViews.org 2012.
Novel, simple, scalable and inexpensive post-treatment to introduce mesoporosity in a wide variety of zeolites - Nanotechnology Solutions for Energy Challenges,
Javier García-Martínez,
ChemViews Mag. 2013.
DOI: 10.1002/chemv.201300086
Javier García-Martínez talks about how simply producing clean energy is not enough and the ways in which nanotechnology can help - Javier García-Martínez on the Commercialization of Research,
Vera Köster,
ChemViews Mag. 2012.
DOI: 10.1002/chemv.201200143
How do you form a spin-off company from your research? Javier García-Martínez talks about his experiences and how he finds time for his many roles - Ending Energy Poverty: Chemistry’s Contribution
Javier García-Martínez,
ChemViews Mag. 2012.
DOI: 10.1002/chemv.201200017
Providing sustainable energy to world’s the seven billion inhabitants is not a small task, but chemistry has a lot to offer, says Javier García-Martínez - Creating a New Generation of Scientists,
ChemistryViews.org 2011.
Javier García-Martínez talks about the four elements of modern chemistry: food, energy, water, climate and how improve life worldwide
.jpg)