Polyether and polyol marine natural products, such as brevetoxins, have extraordinary structures and potent biological activities. Thus, they are attractive molecules in medicinal chemistry. However, their synthesis is challenging due to complex structure features, which include a long carbon backbone that is highly functionalized by oxygen atoms.
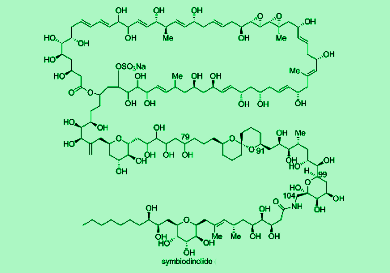
Symbiodinolide is a 62-membered polyol macrolide with a molecular weight of 2860 and 61 stereogenic centers. It displays biological activities at very low concentrations and has been shown to rupture the tissue surface of an acoel flatworm. Hiroyoshi Takamura and co-workers, Okayama University, Japan, first reported the isolation of symbiodinolide from the symbiotic marine dinoflagellate Symbiodinium sp. in 2007. However, the complete configurational elucidation of symbiodinolide still remains an unsolved issue because of its huge and complicated molecular structure. Thus, they report the synthesis of C79-C104 fragment of the symbiodinolide and determine its stereoselectivity using various techniques, such as with the nuclear Overhauser effect (NOE).
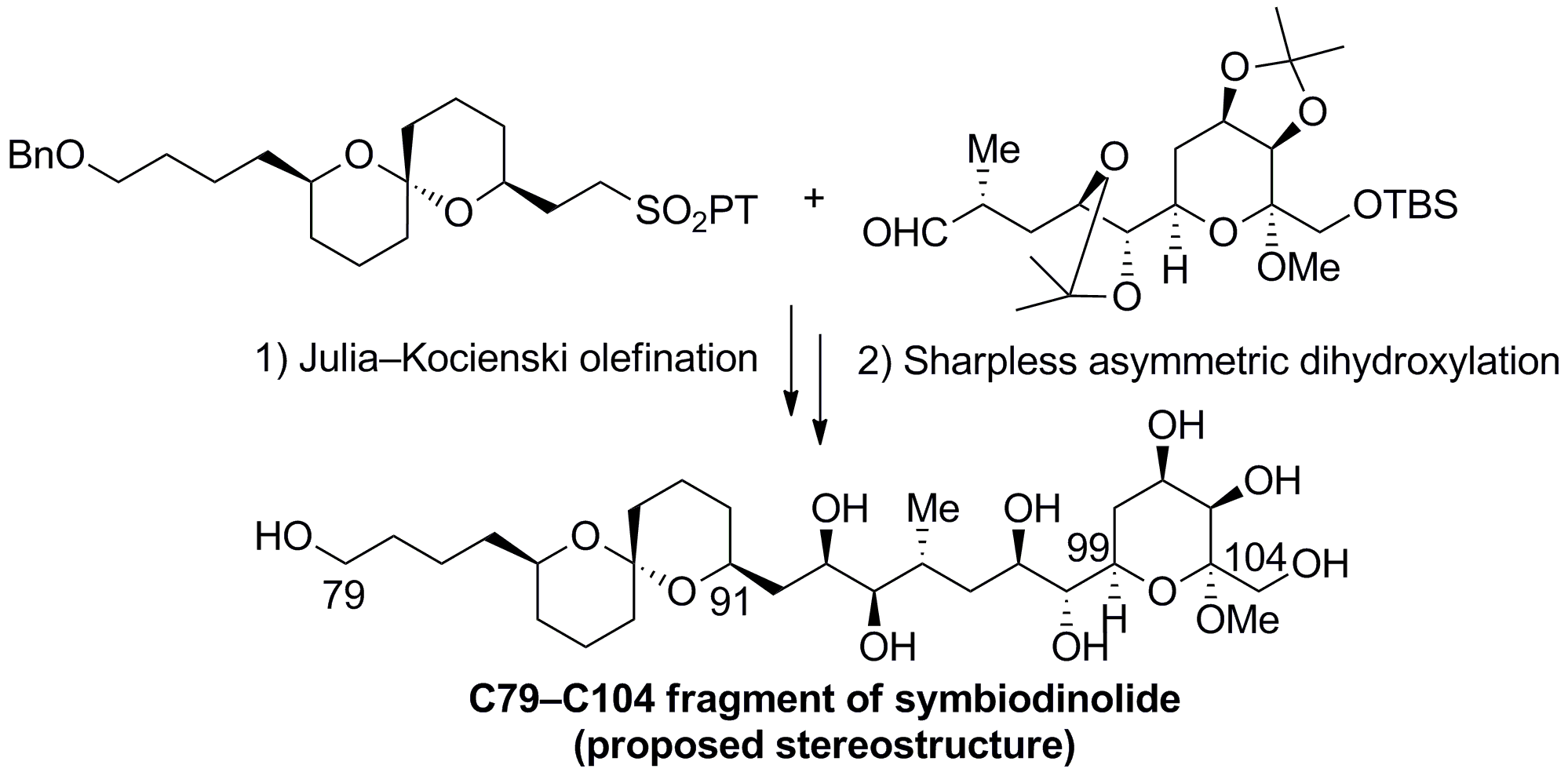
In the synthetic route, the proposed C79-C104 fragment was synthesized by the Julia-Kocienski olefination and subsequent Sharpless asymmetric dihydroxylation as key transformations in a convergent manner. Investigations revealed that the stereochemistry of the C91-C99 carbon domain of the symbiodinolide needs to be reassigned.
- Stereoselective Synthesis of the Proposed C79-C104 Fragment of Symbiodinolide,
Hiroyoshi Takamura, Takayuki Fujiwara, Yohei Kawakubo, Isao Kadota, Daisuke Uemura,
Chem. Europ. J. 2015.
DOI: 10.1002/chem.201503880