The reduction of carboxylic acid esters into the corresponding alcohols is an important reaction in organic synthesis and has extensive practical applications. Compared with traditional stoichiometric procedures using inorganic hydrides, homogeneous catalytic hydrogenation systems are more attractive because of their low cost, high efficiency, and atom economy.
Further improvement of current catalysts and the design of new, cost-effective metal complexes for catalytic hydrogenation of esters rely on a deep understanding of related reaction mechanisms. However, mechanistic insights into catalytic ester hydrogenation reactions are still lacking.
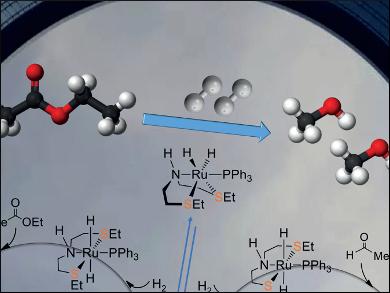
Xinzheng Yang, Beijing National Laboratory for Molecular Sciences, China, and colleagues have proposed and analyzed a new mechanism for the hydrogenation of ethyl acetate into ethanol, catalyzed by Gusev’s SNS ruthenium complexes, using density functional theory (DFT) calculations. Different from a previously proposed mechanism with fac-[(SNS)Ru(PPh3)(H)2] as the catalyst, the mechanism involves an unexpected direct hydride transfer mechanism with a mer-SNS ruthenium complex, with two trans-sulfur atoms in the SNS pincer ligand, as the catalyst. The findings also indicate that the formation of the fac-SNS complex could deactivate the catalytic system.
- Unexpected Direct Hydride Transfer Mechanism for the Hydrogenation of Ethyl Acetate to Ethanol Catalyzed by SNS Pincer Ruthenium Complexes,
Xiangyang Chen, Yuanyuan Jing, Xinzheng Yang,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201504058