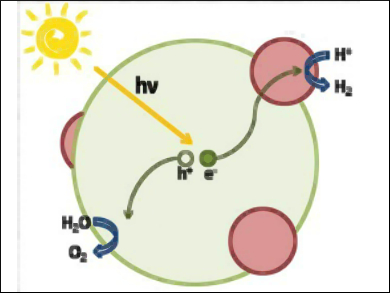
Photoelectrochemical water splitting is an elegant way to transform water into molecular oxygen and hydrogen with the help of sunlight. Water is an abundant resource and hydrogen can directly be used as carbon-free chemical fuel to power a combustion engine or a fuel cell. A major limitation of efficient water splitting lies in finding stable semiconductor photoanodes with suitable bandgaps.
Markus Niederberger, ETH Zurich, Switzerland, and colleagues processed commercially available tungsten oxide nanopowders into nanostructured films and tested them as photoanodes for photoelectrochemical water splitting. The team found that these photoanodes showed high photocurrent densities of up to 3.5 mA/cm at 1.23 V under simulated sunlight. However, further experiments indicated oxidation of the electrolyte rather than of water.
While the study proposes a scalable approach to the fabrication of photoanodes from commercially available WO3 powders, it also indicates that photocurrent density measurements alone are not sufficient to fully evaluate the performance of a photoanode material. These results should have an impact on the technological construction of future water-splitting devices.
- Commercially Available WO3 Nanopowders for Photoelectrochemical Water Splitting: Photocurrent versus Oxygen Evolution,
Sandra Reinhard, Felix Rechberger, Markus Niederberger,
ChemPlusChem 2016.
DOI: 10.1002/cplu.201600241