1. Institute of Science and Technology in Art (ISTA)
Back in 1692, a private art academy was founded under the auspices of the Austrian Emperor Leopold I (1640–1705) and designed by his court painter Peter Strudel (1660–1714). In 1872, Emperor Franz Joseph I of Austria (1830–1916) approved a statute declaring the academy to be the supreme government authority for the arts, with primary subjects such as architecture, sculpture, engraving, metalwork, and painting. Subsidiary subjects include anatomy, perspective and style, as well as color chemistry and theory [1]. In 1950, an associate professorship and institute for color theory, color chemistry, and paint materials was established to educate art students in materials science. Over the years, new areas of institutional focus arose, such as Science in Conservation and Science for Cultural Heritage.
Today the Institute of Sciences and Technology in Art (ISTA) provides a profound theoretical and practice-oriented education in the subjects of color science, theory of perception, materials science, and color chemistry with an increasing emphasis on contemporary materials and technologies. The courses of study do not center merely on scientific theorems and technological models but are rather based on the system of concepts with which artists and students of art are concerned. Unlike other institutes, here art is not dealt with according to aesthetic or stylistic criteria; the curriculum instead focuses on the scientific documentation and materials analysis of objects of our cultural heritage and their specifics, compositions, and stability over time.
In addition to lectures on natural sciences and materials science, studies and research projects are carried out as part of diploma and doctoral theses that deal with scientific investigations of single art objects as well as whole art ensembles. The documentation and investigation of original objects is mainly performed by the application of methods that use electromagnetic radiation (e.g., infrared, visible, UV, and X-ray radiation) and techniques for non-destructive/non-invasive materials analysis. Likewise, research projects explore the long-term behavior of materials (e.g., light stability, corrosion, and weather resistance, as well as the compatibility of various materials used in modern and contemporary art) and also deal with the preservation of historical monuments. In accordance with international trends, these requirements have entailed some new foci in recent years that have been broadly categorized as “Science for Art”, “Science in Conservation and Preservation”, “Science for Cultural Heritage”, and “Heritage Science”.
2. Photographic Documentation and Digital Imaging
To document objects of our cultural heritage, photographic techniques that employ radiation in the visible range (390–780 nm) as well as in the infrared (800–2000 nm), ultraviolet (300–400 nm), and X- or even γ-ray range (<1 nm) are widely used [2, 3]. Such methods enable the visualization of the whole object including those areas normally invisible to the naked eye.
Because photographic techniques are in the strictest sense non-destructive and even non-invasive for art works, they have found early acceptance in stylistic analysis, iconographic and iconological interpretation, social–critical approaches, and in the determination of the materials and state of preservation. Techniques such as X-ray radiography, UV-fluorescence photography, and infrared photography are relatively simple to use and, furthermore, they best fit the way an art historian, social scientist, or conservator would look at a heritage object.
2.1 X-ray Radiography
As can be seen in Figure 1, in ordinary X-ray radiography the radiation transmitted through an entire object—in this case, an easel painting—illustrates a projection of the X-ray absorbing matter present in the support, ground and paint layers. Dense materials such as metals or pigments in both the surface layer and in underlying layers normally not visible can be recorded [4].
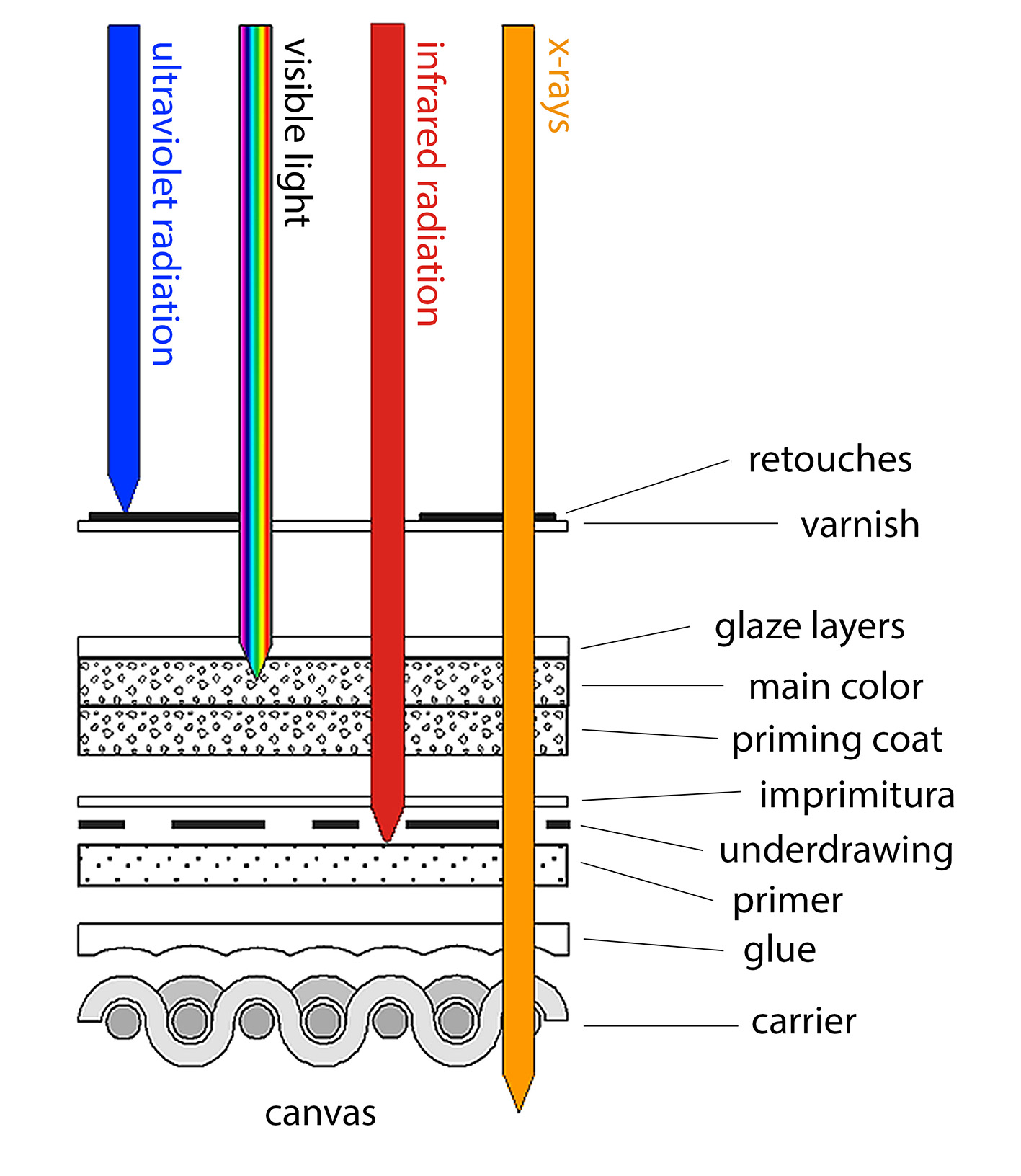
Figure 1. Structure of an easel painting and penetration depths of different types of radiation [4]. © International Center for Diffraction Data, published by Cambridge University Press.
Many case studies are available in the literature in which archaeological findings were investigated and their “inner structure” studied (see Fig. 2) [3]. Additionally, for graphic art, X-ray radiography has been proven to be an important tool for the non-destructive visualization of watermarks owing to the various thicknesses of the paper inside and outside the area of the marks [5].
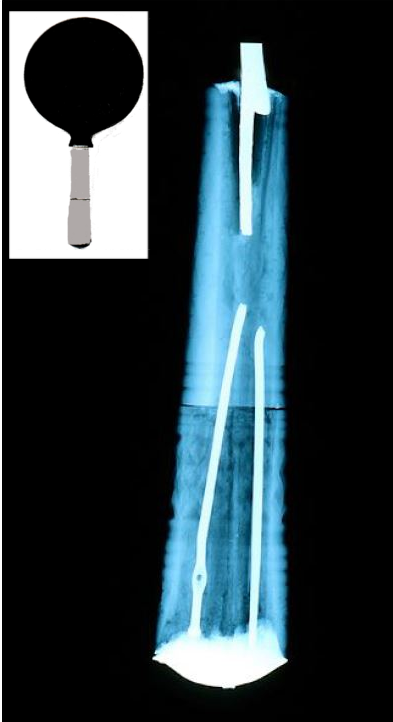
Figure 2. Etruscan mirror (left), 4th century BC, Kunsthistorisches Museum Vienna, Austria, and X-ray radiograph (right) of the handle showing its “inner structure”. Metallic needles were used to strengthen and hold the two parts of the handle together.
2.2 Ultraviolet Photography
Ultraviolet photography has been used to detect discontinuities in the paint surface, especially retouches or later additions (see Fig. 3). Usually, the fluorescence in the visible region of the spectrum excited by UV radiation (see Fig. 1) is photographed. However, UV-fluorescence photography has become a widely used technique for historians, librarians, and paper conservators interested in texts in which the ink has bleached or degraded, or when palimpsests have to be studied (see Fig. 4). A palimpsest is a page from which the text has been scraped or washed off to reuse it for another document.
Figure 3. Gothic Madonna with child after Rogier van der Weyden seen with visible light (left) and under UV-fluorescence (right); Inv. No. A02, The Paintings Gallery of the Academy of Fine Arts Vienna. The dark areas in the UV-fluorescence image result from the materials used for retouches. © The Paintings Gallery of the Academy of Fine Arts Vienna, Austria
Figure 4. Postcard (private) seen in visible light and in UV-fluorescence.
2.3 Infrared Photography and Infrared Reflectography
Infrared Photography
Infrared photography was initially used for the identification of artists’ materials but has subsequently come to be employed almost exclusively for the visualization of underdrawings (see Figs. 1 and 5). Examination of such drawings has yielded clues about artists’ techniques and intentions, the evolution of particular paintings, and the hand of workshop assistants.
The artist—or the members of his workshop—made underdrawings immediately on the ground layer (usually a mixture of white fillers with an aqueous binding medium) to define the form and composition of the presentation. These drawings are on the one hand an early element in the artistic creation but are on the other hand often sketch-like in contrast to the final outlines of the finished painting, as the painters did not always bother to spend much time on their execution given that they were to be covered by superimposing various paint layers. Underdrawings are normally untouched by later hands, in contrast to the surface layers, and provide an unaltered testimony of the artist’s original intention. Therefore, examinations of underdrawings have aided in the attribution, interpretation, and conservation of works of art [6].
Figure 5. Photographic documentation in the visible and infrared range of the easel painting “Unterach” by Gustav Klimt, Österreichische Galerie Belvedere, Inv. No. 4209. In the IR image the underdrawings (concept) of the artist and the pentimenti (red arrows) can be clearly observed. © Belvedere, Vienna, Austria
Infrared Reflectography
However, the study of underdrawings by infrared photography is hampered by the fact that such drawings can only be visualized in part (frequently just in reddish or whitish areas), whereas areas painted, for example, in azurite or greenish colors (with Cu-containing pigments), cannot be investigated. The reason for this is the limited detection of infrared radiation up to approximately 900 or 1100 nm by means of film or charge coupled device (CCD), respectively.
As an alternative technique, so-called infrared reflectography was developed in the 1960s using IR detectors. Van Asperen de Boer [7, 8] applied the Kubelka–Munk theory to the optical properties of paint films and concluded that paints generally display their greatest “hiding thickness” near 2 μm. These results prompted a search for IR-imaging devices. Today the Osiris® camera [9] using an InGaAs detector with a sensitivity up to 1700 nm or even hyperspectral imaging instruments are applied (see Fig. 6) [10, 11].

3. Material Analysis
3.1 History of Material Analysis
Material analysis in the field of cultural heritage has a long history and dates back to the year 1795 when Martin H. Klaproth [12], Professor at the University of Berlin, Germany, published research on the material composition of Roman coins, ancient metal alloys, and archaeological glasses. Objects had to be dissolved in hydrochloric and nitric acids and analytical procedures had to be developed by the chemists for separating the constituents, such as copper from zinc or tin, to achieve quantitative results. A big step forward was the development of microchemical spot tests [13], so testing on very small amounts of sample(s), which reduced the amount of sample material necessary for analysis.
Consequently, the museum’s first laboratories were installed in the early decades of the last century and the booming development of analytical methods for material identification has brought a great number of new instrumental microanalytical techniques with non-sampling (without taking original sample material) and in situ applicability to an artefact. Most of these new instruments can be used in air. In most cases the analysis is non-destructive or even non-invasive. As a consequence to the miniaturization in the field of electronics yielded devices, the instruments can easily be transported to an archaeological site or into museums, libraries, and galleries for the determination of the material composition of a heritage object.
3.2 Interdisciplinary Cooperations
Therefore, it is not surprising that in recent decades a particular collaboration between historical, philological, and cultural studies on the one side and natural sciences on the other has been developed and a close cooperation between archaeology, art history, or conservation–restoration with physics, chemistry, or biology, for example, seems fairly well established. One reason for this is that art historians, conservators, and archaeologists are most constantly concerned with the questions of where, when, and by whom an object of art or archaeology was made. Investigations of the physical properties and the material composition are used increasingly in addition to the visual and stylistic analyses, to allocate an object to a particular historic or prehistoric context, to determine the correctness or incorrectness of the claimed provenance, or to explore the technology used for manufacturing an object.
The basis, therefore, is to find out when new materials, techniques, or technologies were introduced or became out of use. However, the analysis of a great number of dated or precisely dateable artefacts is necessary and enables us to set up a “terminus post quem” or a “terminus ante quem” for pigments used for paintings or metal alloys for coins, for example. The same is true for methods developed or even invented for materials processing or fabrication.
Another reason for this close cooperation between chemistry and cultural heritage disciplines, which might also help determine the authenticity of an art work, concerns the present state of objects. Scientists working in the field of art and cultural heritage are confronted more and more with deterioration, and, hence, restoration and preservation. As material degradation/corrosion under natural conditions differs in many cases from artificial aging, the determination of corrosion products and processes can also be used for the identification of authentic artwork.
3.3 Analytical Methods Used Today
Among the great variety of instrumental techniques that have been applied to study the elemental composition of objects of art and archaeology, X-ray fluorescence analysis (XRF) using X-, γ-, or synchrotron radiation as well as particles such as protons has attracted growing interest [14, 15]. The great advantage of energy-dispersive XRF is that in principle all elements (except hydrogen and helium) of the periodic table can be detected. Furthermore, handheld instruments are now readily available that can be used on-site.
Unfortunately, when using XRF in air, only elements with an atomic number higher than 16 (sulfur) can be detected, which limits the characterization of an art work to its inorganic components. Furthermore, as only the elements present can be determined, no compound-specific information is obtained. Therefore, in the case of calcium carbonate (CaCO3), for example, no differentiation between the two modifications calcite and aragonite is possible. Also mixtures of inorganic pigments can rarely be completely characterized; for example, the detection of lead (Pb) in a red paint layer indicates the presence of red lead (Pb3O4), but mixtures of this red pigment with lead white (lead carbonate, PbCO3) cannot be clearly identified by using just XRF.
For these reasons, compound-specific methods such as Fourier transform infrared (FTIR) [16] and Raman spectroscopy as well as spectrometry in the UV and visible range (UV/Vis) [17] are promising complementary techniques, as such measurements can also be carried out in air and in a non-invasive way. FTIR spectroscopy in the transmission mode, for which small samples are required, has been used for decades for the identification of organic as well as inorganic materials in art and archaeology in those cases where sampling was possible [18]. In recent years, reflection-FTIR spectroscopy for non-invasive analysis has been described in the literature using mirrors or fibers for controlling the optical path from the instrument to the object and vice versa [19, 20].
3.4 Methodes Used at ISTA
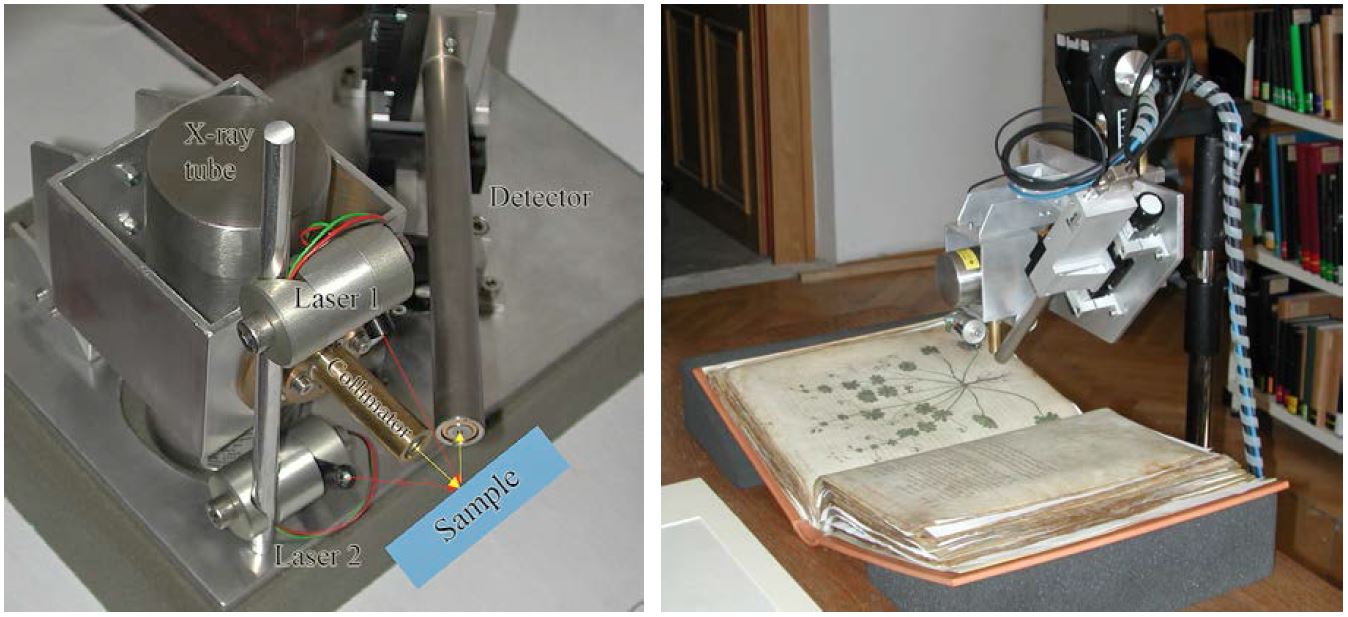
At ISTA we focus on transportable instruments for XRF as well as UV/Vis, FTIR, and Raman spectroscopy [21], for which new setups have been designed and assembled, to permit in situ examinations in museums, libraries, and even at archaeological excavation sites [22, 23]. The XRF system is based on energy-dispersive XRF using an Oxford 50 W rhodium X-ray tube (type XTF5011), a Röntec silicon drift-chamber detector (type XFlash 1000), and two lasers for positioning (see Fig. 7). The crossover of the two laser beams (red in Fig. 7) coincides with the crossover of the axes of the primary X-ray beam and the detector (yellow).
Figure 7. Transportable XRF system consisting of an X-ray tube with a collimator and a silicon-drift-chamber detector (left); and during analysis of the famous Viennese Dioscurides, Greek, 5th century AD in the Austrian National Library, Vienna (right). © ISTA
The system for UV/Vis spectroscopy consists of a 75 W xenon short-arc lamp (Ushio Inc., Japan), which is housed in a monochromator (type CLX 75-2 of J&M Analytik AG, Aalen, Germany). Two quartz fiber optic cables (1250 µm core diameter, length 2 m, J&M) direct the beam from the source to the measuring point on the object and from the object to the spectrometer. The utilized spectrometer (MSP 400, J&M) is equipped with a 256 diode-array detector that allows measurements in the range 300–1150 nm. The fiber optic cables are fixed to a measuring head in 0°/45° geometry by a self-built component that was constructed for a measuring distance of 5 mm between the fiber optics and the object (see Fig. 8).

Figure 8. Custom-built UV/Vis system for microscopic and non-invasive color measurements (left) and fiber optic measuring head (right). The main construction elements are as follows: 0°/45°-mounting for the quartz fiber optic cables (a), a collimator made from latex (b), a laser pointer for precise positioning (c), a battery compartment (d), a switch for lasers on/off (e), and the x-z-axial positioning mechanism (f). Red lines symbolize the laser beams. © ISTA
For the compound-specific FTIR analysis, an external reflection unit (Bruker Optics, Ettlingen, Germany) is usually applied. The reflection module, which can be mounted onto the portable FTIR spectrometer, focuses the IR beam onto the object by using mirrors. The analyzed area is in the range of approximately 4 mm in diameter. The reflected radiation (4000–400 cm–1) is focused again by mirrors to the DTGS-detector (pyroelectric detector with deuterated triglycinsulfate as detector material) and the spectrum obtained has to be mathematically treated by using the Kramers–Kronig transformation (KKT) to achieve a so-called absorption index spectrum, which can be compared with an IR spectrum of a database obtained in the transmission mode [24].
3.5 Example: Pigment Analysis in Medieval Miniature Paintings on Parchment
Triumphal processions have a long tradition from ancient periods to modern times and it is not surprising that a depiction of such was made for the Roman Emperor Maximilian I (1459–1519; see a picture at: thehistoryblog.com). This object originally consisted of 109 large parchment sheets with flamboyantly colorful representations of riders, magnificent chariots, and landsknechts. Sheets 49 to 109 are preserved in the Albertina in Vienna, Austria, and, compiled as a frieze, amount to a length of more than 54 m. During conservation–restoration treatment of these miniature paintings, pigment analyses were also carried out, and the results are summarized in Table 1.
In most cases the pigments could be identified by their elemental composition and their colors. However, in a few cases no elements characteristic for a pigment could be detected or—in case of the green parts of the paintings—several pigments containing copper do exist and were used during the period of the painter Albrecht Altdorfer.
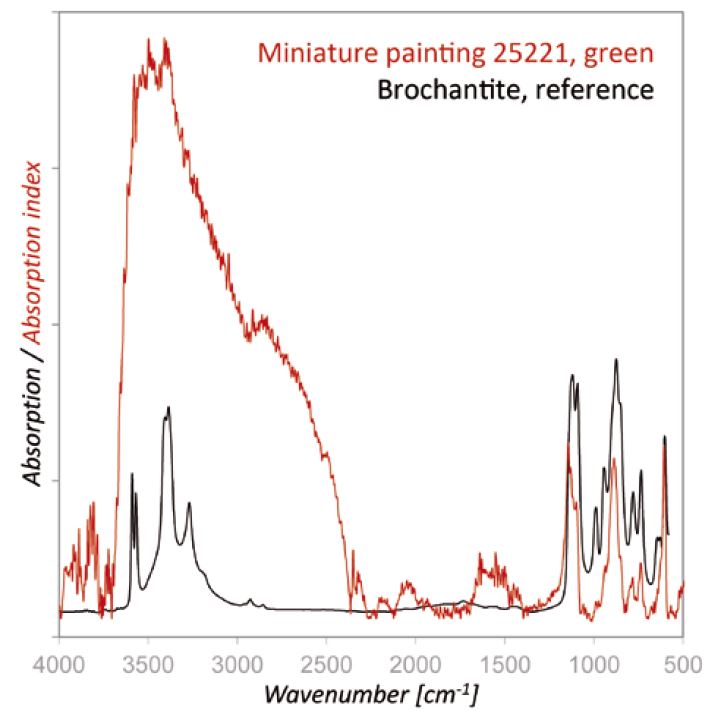
Compound-specific analysis by r-FTIR (FTIR in the reflection mode with the Bruker Alpha system) yielded the spectrum presented in Figure 9. By comparing the absorption index spectrum (after Kramers–Kronig transformation) with the Infrared and Raman Users Group (IRUG) database [24], good agreements with the spectrum of brochantite were found, which was obtained by measurements in the FTIR transmission mode.
Table 1. XRF results of the pigments in “The Triumphal Procession of Emperor Maximilian I“.
|
Color |
Element(s) detected |
Interpretation |
| Gold | Au | pure gold |
| White | Pb | lead white |
| Yellow | Pb, Sn | lead-tin-yellow |
| S, As | arsenic sulfide: orpiment or realgar | |
| Orange | (S), Fe, As | mixture of ochre and arsenic sulfide |
| Red | (S), Hg | cinnabar or vermillion |
| Blue | Cu | azurite |
| Green | Cu, (Pb) | malachite, verdigris ??? |
| Dark red (violet) | – – – | madder ??? |
| Dark blue | Pb, – – – | indigo ??? mixed with lead white |
| Brown | – – – | organic material ??? |
| Hg, Pb | mixture of cinnabar + lead white ??? |
Figure 9. r-FTIR spectrum of the green areas of the painting The Triumphal Procession of Emperor Maximilian I, which shows the typical bands of brochantite.
3.6 Example: Watercolors of the 19th Century
Several important Austrian painters of the 19th century used watercolors as their preferred medium, for example, Rudolf von Alt (1812–1905) and Moritz Michael Daffinger (1790–1849). The Kupferstichkabinett (Graphic Collection) of the Academy of Fine Arts, Vienna, holds a large number of works of these painters (see Fig. 10), thus it is important to know which materials have been applied and how they will change over the years. These questions have been studied by using X-ray fluorescence analysis, infrared, and UV/Vis spectroscopy.
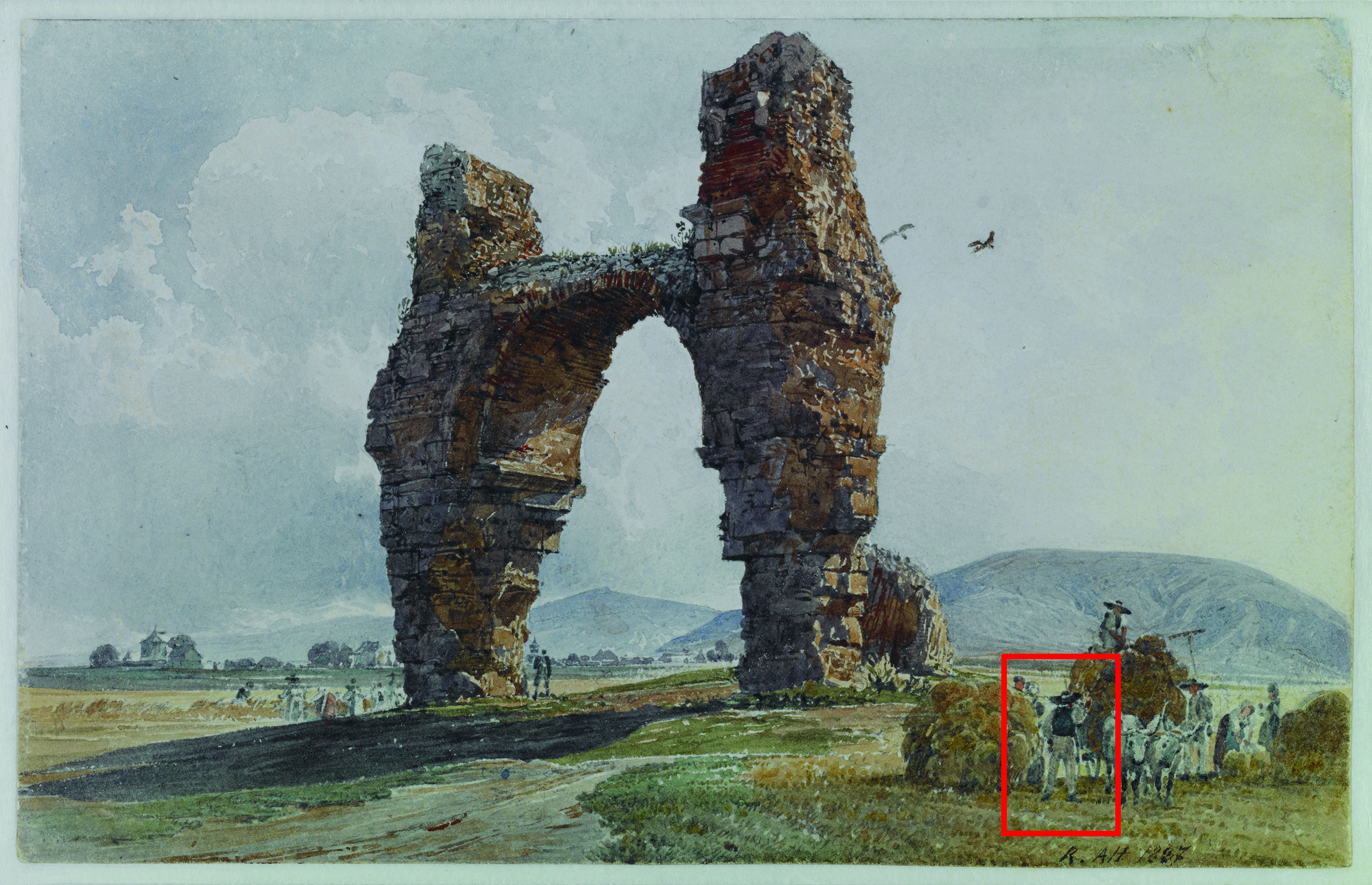
Figure 10. Watercolor by Rudolf von Alt “Das Heidentor bei Petronell”, 1837; Graphic Collection of the Academy of Fine Arts Vienna, Inv. No. HZ 13010. © Kupferstichkabinett, Academy of Fine Arts Vienna, Austria.
Owing to the combination of different analytical techniques, the identification of both inorganic and organic components was possible for most of the paintings analyzed. Zinc white, ultramarine blue, ochre, and vermillion were identified in the paintings. As iron could be detected by XRF in the blue areas, the conclusion could have been drawn that Prussian blue was applied by the painter in these areas. However, the UV/Vis spectrum (see Fig. 11) clearly depicted the remission curve of indigo, which might have been mixed with small amounts of ochre, an iron-containing silicate.
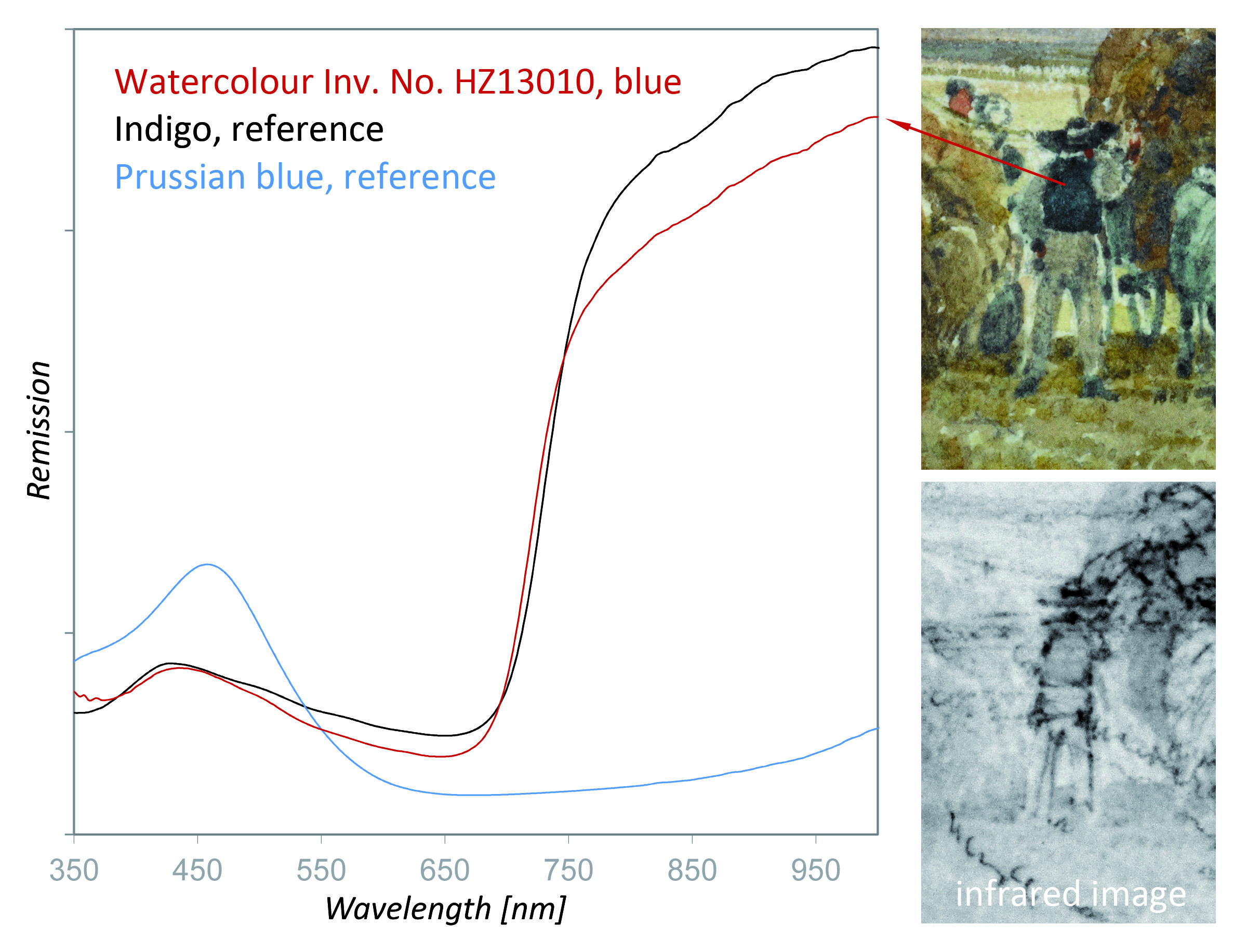
Figure 11. Identification of indigo in the watercolor by using UV/Vis spectroscopy in the reflection mode. In contrast to Prussian blue, indigo does not absorb near-infrared radiation and appears light in the infrared image.
4 Material Degradation
4.1 The Stability of Materials
Scientific investigations are indispensable for studying material degradation processes on heritage objects. Art works as well as archaeological findings made of glass or metals such as medieval stained glass or outdoor bronzes suffer a great deal of damage due to their exposure to the ambient atmosphere or soil over centuries, and in some cases even for millennia. The present state of such objects is highly influenced by environmental conditions. Therefore, the degradation behavior is worth investigating and is in fact indispensable for developing strategies and methods to reduce or even prevent such corrosion.
Furthermore, for synthetic organic materials in contemporary art, its light stability has to be considered from a scientific point of view. To ensure protective and secure conservation of cultural assets in their original state, highly sensitive analytical methods are used to investigate the dynamic chemistry of the atmospheric deterioration behavior of various materials used in the arts. An increasingly important part of our research focuses, therefore, on the stability of materials and their long-term behavior. Investigations have been performed to study the degradation behavior of objects of metal, glass, or enamel [25–27]. Additionally, the light stability of pigments, dyes, and polymers in contemporary art has been investigated.
4.2 Dynamic Degradation Studies of Metals and Polymers
It has become a main goal of scientists, conservators, and archaeologists to rescue, preserve, restore, and increase public awareness of the historical, archaeological, and cultural heritage. An important part of this research consists of identifying the mechanisms responsible for the degradation of materials. As objects undergo degradation as a consequence of exposure to certain atmospheres, it is of special interest to clarify the occurring reactions by using in situ, time-lapse chemical and structural analysis in controlled ambient atmospheres to develop methods and strategies to reduce or even prevent the atmospheric attack [28–31].
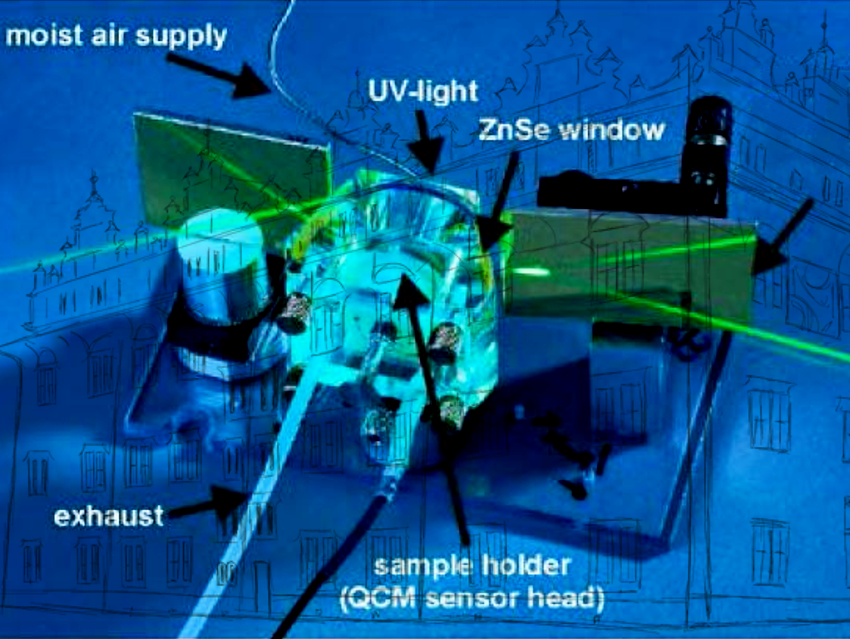
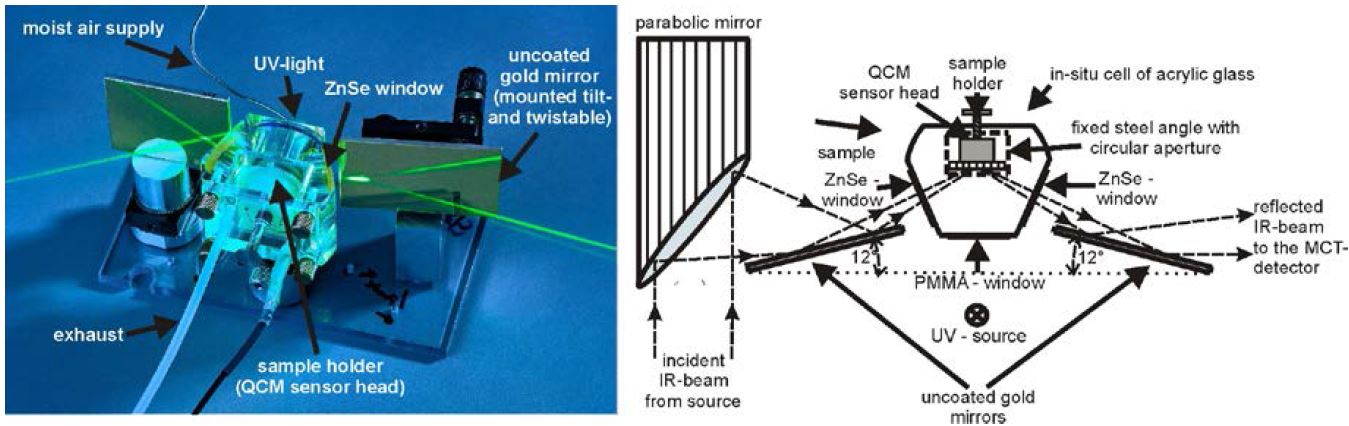
To ensure protective and secure conservation of cultural assets in their original state, highly sensitive analytical methods such as in situ infrared reflection absorption spectroscopy (IRRAS), quartz crystal microbalance (QCM), Raman spectroscopy, atomic force microscopy (AFM), scanning electron microscopy (SEM), Kelvin probe (KP), and X-ray diffraction (XRD) can be applied to investigate the dynamic chemistry of the atmospheric degradation behavior of different materials while exposed to controlled humidity, ozone, and acidifying gases. For this reason a special setup – a weathering cell as shown in Figure 12 – was designed and built. It allows simultaneous in situ time-lapse IRRAS and QCM degradation studies [32].
Figure 12. Scheme and image of the weathering cell for simultaneous in situ IRRAS/QCM measurements. The unit consists of an acrylic glass body with gas in- and outlet, two ZnSe windows, and a polymethylmethacrylate window for the UV irradiation. In the center of the unit is the sample holder with the integrated quartz crystal microbalance (QCM) sensor head. With this setup it is possible to weather samples at any defined atmosphere with addition of different acidifying gases; furthermore, the sample can be irradiated with UV light.
4.2.1 The Weathering Cell: Degradation of Pure Silver
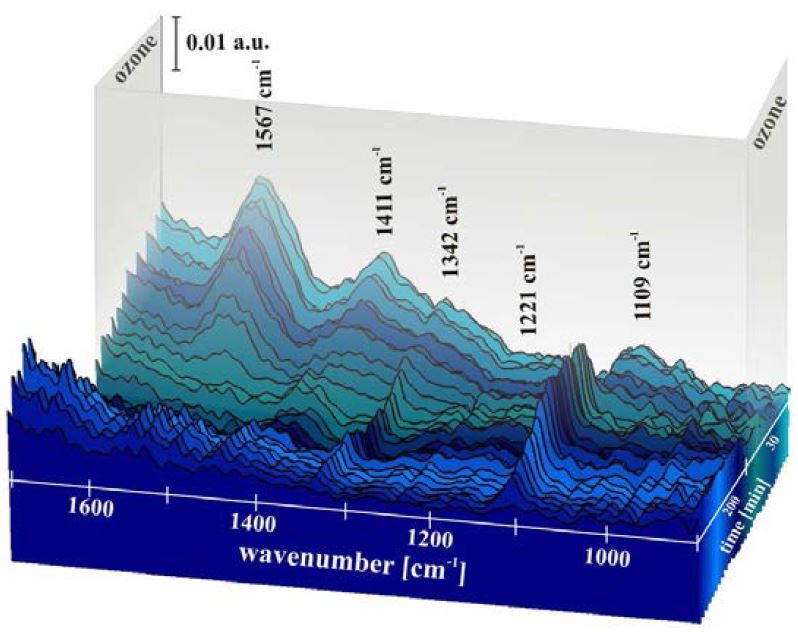
The potential of the weathering cell for studying time-lapse degradation processes is shown in Figure 13. Extremely pure silver was exposed to 90 % relative humidity (RH), room temperature (RT), and 500 ppm CO2 for the first 200 min followed by the addition of 200 ppb O3 to this atmosphere for 30 min. IR spectra were automatically collected every 10 min. During weathering, the growth of absorption bands can be observed at the following wavenumbers: 1342 (strong), 1221 (weak), and 1109 cm−1 (strong).
The 1221 and 1109 cm−1 bands can be correlated to the –C–O asymmetric stretch vibration and the asymmetric CO32− stretch vibration of basic silver carbonate (AgOH∙Ag2CO3) surface species. The band at 1342 cm−1 has been assigned to the absorption frequency of the CO2 symmetric-stretching vibration in basic surface carbonates. Therefore, it can be concluded that on silver surfaces exposed to CO2 and RH, basic silver carbonate is formed, whereas in the presence of O3 (in addition to CO2) the formation of a complex carbonate surface species occurs at the expense of the basic silver carbonate species [33].
Figure 13. In situ time-resolved IRRAS spectra of a silver sample exposed to 90 % RH and 500 ppm CO2 for the first 200 min followed by addition of 200 ppb O3 to this atmosphere for 30 min.
4.2.2 The Weathering Cell: Degradation of Synthetic Polymers
Another application of the weathering cell is degradation studies of synthetic polymers such as polyester or epoxy resins. They play an important role in modern artists’ materials with widespread applications, including as stabilizing and coating composites, for metals, wood, plastics, photographs, paints, inkjet prints, canvases, inks, papers, and spray paints.
Surface-sensitive analytical methods have been applied to investigate the degradation behavior of polyester and epoxy resins exposed to controlled atmospheres containing synthetic air, 70 % RH, SO2, and H2S in the parts per million range, as well as O3 in the parts per billion range [34]. For these investigations, the custom-made in situ weathering chamber was used to track chemical reactions and gravimetric changes during weathering. Figure 14 (upper graph) shows the time-lapse IRRAS measurements of polyester resin exposed to synthetic air, 70 % RH, and 2.4 H2S (ppm) for 90 hours. For a better estimation of small changes of absorbance bands over time the time-lapse spectra were all divided by the spectrum obtained at time 0 (reference).
By evaluating the increase or decrease of the absorbance bands, which are related to certain chemical species, conclusions can be drawn about the degradation behavior of these resins under different atmospheric conditions. These fundamental deterioration studies are indispensable for developing strategies and methods to reduce such environmental attack.
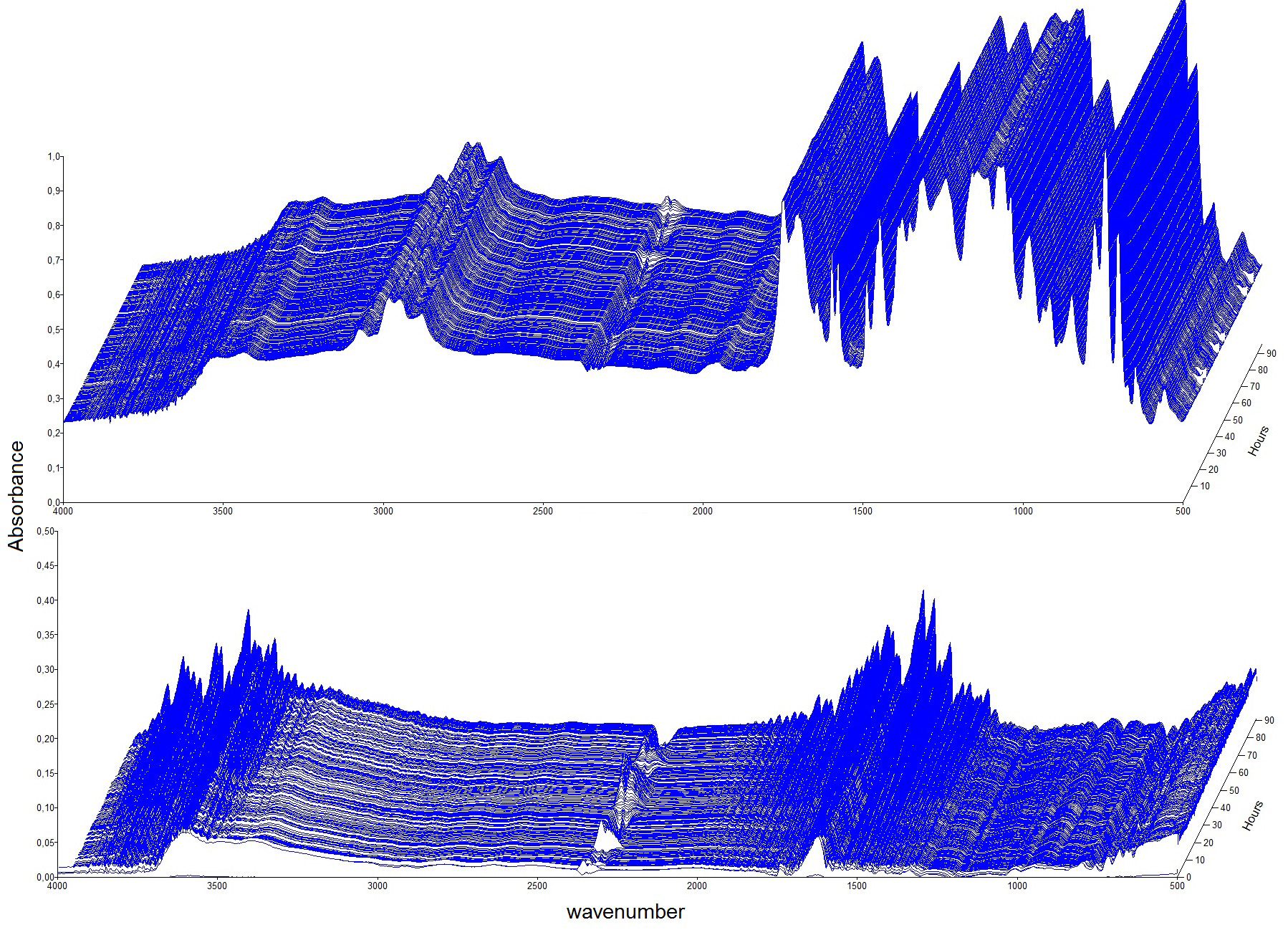
Figure 14. Time-lapse IRRAS measurements of polyester resin exposed to synthetic air, 70% RH, and 2.4 ppm H2S for 90 hours (upper graph). The lower graph shows the time-lapse spectra after calculating the ratio by using the measurement at time 0 as a reference. © ISTA
4.3 Long-Term Metal Corrosion
Within the framework of the Working Group on Effects on Materials Including Historic and Cultural Monuments of the United Nations Economic Commission for Europe (UN/ECE), the effects of various air pollutants (such as SO2, NOx, and O3) as well as climatic parameters (temperature, relative humidity, amount of precipitation) on the atmospheric corrosion (weathering) of various materials has been studied [34]. Since 2008 the ISTA has provided an exposure site in cooperation with the Central Institute for Meteorology and Geodynamics (ZAMG, Vienna, Austria).
Samples of zinc, steel, aluminium, copper, sandstone as well as modern glasses provided by international partners in the UN/ECE project were exposed on a wooden rack (see Fig. 15) for exposure times of between one and seven years. Environmental and climatic data were measured and provided by the ZAMG.
The aim of this project, which has existed since the mid-1980s, is the development of so-called dose-responsive functions expressing the mathematical relation between the amount of corrosion of the materials and the environmental conditions on-site. These functions can be used to predict the damage by air pollution to objects of our cultural heritage and for the assessment of threshold levels for the pollutants to keep the atmospheric corrosion at an “acceptable” level.

Figure 15. Exposure site in cooperation with the Central Institute for Meteorology and Geodynamics (ZAMG) after mounting the samples (left) and after one year of exposure (right). © ISTA
Authors
Professor Dr. Manfred Schreiner1,2, Dr. Rita Wiesinger1, and Dr. Wilfried Vetter1
1) Institute of Science and Technology in Art, Academy of Fine Arts, Schillerplatz 3, 1010 Vienna, Austria
2) Institute of Chemical Technologies and Analytics, Vienna University of Technology, Getreidemarkt 9/164, 1060 Vienna, Austria
Acknowledgement
The authors would like to gratefully acknowledge the cooperation and the fruitful discussions with the directors, curators, and conservators of the various museums and libraries. Special thanks are also due to Ernst-Georg Hammerschmid and Dr. Michael Melcher for their invaluable help preparing the figures and the manuscript for publication.
5 References
[1] Die Geschichte der Akademie der bildenden Künste in Wien, W. Wagner, Brüder Rosenbaum, Vienna, Austria, 1967.
[2] Strahlenuntersuchung an Kunstwerken, F. Mairinger, E. A. Seemann Verlag, Leipzig, Germany, 2003. ISBN 3-363-00778-7
[3] Radiography of Cultural Material, J. Lang, A. Middleton, Butterworth-Heinemann, Oxford, UK, 1997. ISBN 0-7506-6347-2
[4] M. Schreiner, B. Frühmann, D. Jembrih-Simbürger, R. Linke, X-rays in Art and Archaeology: An Overview, Powder Diffr. 2012, 19 (1), 3–11. DOI: 10.1154/1.1649963
[5] Determination of Watermarks by Non-Destructive Techniques, Comparative Studies in: Paper as a Medium of Cultural Heritage, M. Schreiner, H. Wallner-Holle, Istituto centrale per la patologia del libro, Rome, Italy, 2004, 142–152.
[6] A. Philipart, R. Van Schoute, Bibliographie de l’infrarouge et du dessin sous-jacent 1986-1988, Le dessin sous-jacent dans la peinture, Colloque VII, Université Catholique de Laovain, 1989, 175–210.
[7] Infrared Reflectography, J. R. J. Van Asperen de Boer, Thesis, University of Amsterdam, 1970.
[8] Infrared Reflectograms of Panel Paintings, J. R. J. Van Asperen de Boer, Stud. Conserv. 1966, 11, 45–46.
[9] http://www.opusinstruments.com/osiris/
[10] Improved Visualization of Underdrawings with Solid-State Detectors Operating in the Infrared, E. Wamsley, C. Metzger, J. K. Delaney, C. Fletcher, Stud. Conserv. 1994, 39, 217–231.
[11] Elucidating Reflectograms by Superimposing Infrared and Color Imaging Devices, D. Saunders, J. Cupitt, National Gallery Technical Bulletin 1995, 16, 61–65.
[12] M. H. Klaproth, Abhandlungen der königlichen Akademie der Wissenschaften und schönen Künste zu Berlin, Teil Experimental-Philosophie 1792–1797, 3–14, 72–8.
[13] Spot Test in Inorganic Analysis, F. Feigl, Elsevier, Amsterdam, The Netherlands, 1954;
Spot Tests in Organic Analysis, F. Feigl, Elsevier, Amsterdam, The Netherlands, 1954.
[14] M. Mantler, M. Schreiner, X-ray Fluorescence Spectrometry in Art and Archaeology, X-ray Spectrom. 2000, 29, 3–17.
[15] Non-destructive Microanalysis of Cultural Heritage Materials, K. Janssens, R. Van Grieken (Editors), Elsevier, London, UK, 2004. ISBN: 0-444-50738-8
[16] Fourier Transform Infrared Spectrometry, P. R. Griffiths, J. A. de Haseth, J. D. Winefordner (Editor), 2nd edition, Wiley, Hoboken, NJ, USA, 2007. ISBN-13: 978-0471194040
[17] Color Science in the Examination of Museum Objects: Non-destructive Procedures, R. Johnston-Feller, T. Ball, E. Maggio (Editors), The J. Paul Getty Trust, Los Angeles, USA, 2001. ISBN-13: 9780892365869
[18] Scientific Tools for Conservation: Infrared Spectroscopy in Conservation Sciences, M. A. Derrick, D. Stulik, J. M. Landry, The J. Paul Getty Trust, Los Angeles, CA, USA, 1999. ISBN: 0-89236-469-6
[19] Characterization of Pigment-Binding Media Systems: Comparison of Non-invasive In Situ Reflectance FTIR with FTIR Microscopy, W. Vetter, M. Schreiner, ePS (e-PreservationScience) 2011, 8, 10–22. http://www.morana-rtd.com/e-preservationscience/2011/Vetter-12-07-2010.pdf
[20] Coloring Materials of Pre-Columbian Codices: Non-Invasive In Situ Spectroscopic Analysis of the Codex Cospi, C. Miliani, F. Rosi, A. Burnstock, B. G. Brunetti, A. Sgamelotti, J. Archaeol. Sci. 2012, 39 (3), 672–679.
[21] http://www.ntk.akbild.ac.at/
[22] A LabVIEW-Controlled Portable X-ray Fluorescence Spectrometer for the Analysis of Art Objects, V. Desnica, M. Schreiner, X-ray Spectrom. 2006, 35, 280–286. DOI: 10.1002/xrs.906
[23] A Fiber Optic Reflection-UV/Vis/NIR-System for Non-destructive Analysis of Art Objects, W. Vetter, M. Schreiner, Advances in Chemical Science 2014, 3 (1), 7–14. http://www.seipub.org/acs/paperInfo.aspx?ID=11143
[24] IRUG database 2007: http://www.irug.org
[25] Leaching Studies of Potash–Lime–Silica Glass with Medieval Composition by IRRAS, M. De Bardi, R. Wiesinger, M. Schreiner, J. Non-Cryst. Solids 2013, 360, 57–63.
[26] ToF-SIMS Analysis for Leaching Studies of Potash–Lime–Silica Glass, M. De Bardi, H. Hutter, M. Schreiner, Appl. Surf. Sci. 2013, 282, 195–201.
[27] Glass of the Past: The Degradation and Deterioration of Medieval Glass Artifacts, M. Schreiner, Microchim. Acta 1991/II, No.1–6, 255–264.
[28] New Trends in Analytical, Environmental, and Cultural Heritage Chemistry, M. P. Colombini, L. Tassi, Transworld Research Network, Kerala, India, 2008.
[29] Basic Environmental Mechanisms Affecting Cultural Heritage, D. Camuffo, V. Fassina, J. Havermans, kermesquaderni, 2010.
[30] Atmospheric Corrosion, C. Leygraf, T. E. Graedel, Wiley, New York, USA, 2000.
[31] Atmospheric Chemical Compounds: Sources, Occurrence and Bioassay, T. E. Graedel, D. T. Hawkins, L. D. Claxton, Academic Press, Orlando, USA, 1986.
[32] A New Experimental Setup for In Situ Infrared Reflection Absorption Spectroscopy Studies of Atmospheric Corrosion on Metal Surfaces Considering the Influence of Ultraviolet Light, R. Wiesinger, C. Kleber, J. Frank, M. Schreiner, Appl. Spectrosc. 2009, 63, 466–470.
[33] Investigations of the Interactions of CO2, O3, and UV Light with Silver Surfaces by In Situ IRRAS/QCM and Ex Situ TOF-SIMS, R. Wiesinger, M. Schreiner, C. Kleber, Appl. Surf. Sci. 2010, 256, 2735–2741.
[34] International Co-operative Programme on Effects on Materials including Historic and Cultural Monuments (ICP Materials), Kista, Sweden