Criegee intermediates (CIs) are highly reactive species formed in alkene ozonolysis, an important reaction in the atmosphere. Reactions of CIs with trace gases can lead to aerosol particle formation, which affects air quality and climate. For small CIs, reaction with water vapor is considered the major sink, while larger CIs tend to thermally decompose. These sinks are strongly dependent on local conditions of temperature and relative humidity.
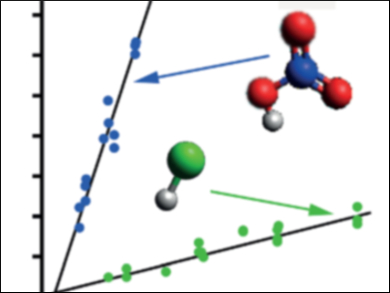
Craig Murray and colleagues, University of California, Irvine, USA, measured the rates of reactions of the simplest CI, CH2OO, with HCl and HNO3, which are present as trace gases in the atmosphere. After photochemically generating CH2OO in a flow reactor, the researchers used UV LEDs to measure transient absorption spectra that show how the CH2OO loss rate varies with the concentration of acid. The reaction with HNO3 is particularly fast, and is likely competitive with other known sinks.
The results show that trace species may be important sinks for CIs where local conditions mean low concentrations of water vapor (lower temperatures or relative humidity), or high concentrations of other trace gases (HNO3 is particularly prevalent in polluted urban areas, such as the Southern California Air Basin).
- Reactions between Criegee Intermediates and the Inorganic Acids HCl and HNO3: Kinetics and Atmospheric Implications,
Elizabeth S. Foreman, Kara M. Kapnas, Craig Murray,
Angew. Chem. Int. Ed.2016.
DOI: 10.1002/anie.201604662