Photons and Plasmons
Just as photons are bundles of light energy, plasmons are energy packets of plasma oscillations—oscillations of the electron density in a solid body, which are known as surface plasmons when occurring at a metal interface. Surface plasmons introduce new possibilities for the manipulation and transmission of light for applications in a variety of areas, from modern data processing to biomedical sensing. Thomas W. Ebbesen, James A. Hutchison, and a team from the University of Strasbourg, France, introduce an interesting effect based on the coupling of photons and plasmons: dye molecules help light pass through holes in metal foils that are so small that conventional theory predicts the light should not actually be able to pass through at all.
Contradicting Classical Theory
According to classical aperture theory, light should not be able to pass through tiny holes when the diameter is significantly smaller than the wavelength of the light. However, as reported by Ebbesen’s group over a decade ago, light transmission can be much higher than predicted for regular arrays of holes owing to the involvement of surface plasmons. In essence, light is converted into surface plasmons, and in this coupled state the photons can pass though the holes to the other side of the metal as plasmons. They can then uncouple and reappear as light.
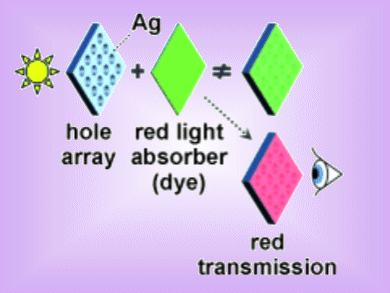
The French team has now described another phenomenon: if dye molecules are placed directly on the perforated metal surface, they significantly increase its transparence. Contrary to expectation, the additional windows of transparency can occur at wavelengths that are strongly absorbed by the molecules. Interestingly, this also occurs if the arrangement of holes in the foil is irregular; even a single hole is enough.
Tailored Transmission
The researchers propose that two complementary effects are at play. On one hand, the dye molecules in the holes generate a large index variation in the hole favoring the transmission near the absorption band. On the other, the dye molecule generates a kind of “mirror image” of its electric dipole in the metal’s free electron plasma, and the dipole and mirror-image dipole interact. If the molecule then absorbs light, it is not re-emitted; instead, the light energy is completely transferred to the metal surface, where it couples with surface plasmons helping the transmission process. This combination enables the light to pass efficiently to the other side of the metal foil.
This discovery represents a new approach for making perforated metal films with tailored transmission of visible light by simply applying a dye that absorbs light with the desired wavelength, which would have application in solar energy technology, filters, and sensing. That the transient excited states of molecules have absorption properties that are very different to their ground state adds a further dynamic dimension to these films, with all-optical, ultra-fast switches another possible application.
- Absorption-Induced Transparency
J. A. Hutchison, D. M. O’Carroll, T. Schwartz, C. Genet, T. W. Ebbesen,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201006019