Graphene, a two-dimensional allotrope of carbon, is the subject of intense research because of its useful properties. Much less is known about other 2D elemental materials. Antimonene, for example, a group-15 2D material, has been predicted to have properties such as high thermal conductivity and carrier mobility. Its synthesis, however, is challenging. Previous approaches have used molecular beam epitaxy or mechanical exfoliation, but suffer from small yields or very small domains of product, which make it difficult to study the material in depth.
Haibo Zeng, Nanjing University of Science and Technology, China, and colleagues have developed a simple synthesis for high-quality single-crystal antimonene. The team used a van der Waals epitaxy growth approach, where thin films of antimonene are vapor-deposited on a mica substrate. The technique allows the growth of materials on substrates with mismatched crystal lattices because the layers only weakly interact through van der Waals forces. Antimony powder was heated to 660 °C to produce antimony vapor, which was then deposited on a fluorophlogopite mica (KMg3(AlSi3O10)F2) substrate heated to 380 °C over a period of 60 minutes.
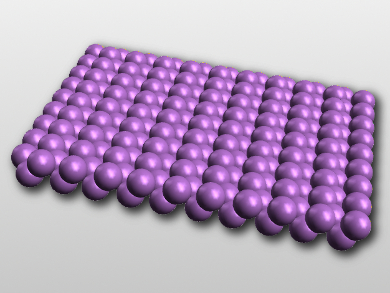
The researchers found antimonene sheets with polygonal shapes (e.g., triangles, hexagons, and trapezoids) with sizes of 5–10 μm and thicknesses about 1–4 nm. The antimony atoms in the crystalline layers form a buckled rhombohedral structure consistent with β-phase antimony (pictured). The material is stable in air for 30 days and shows high electrical conductivity and optical transparency. According to the researchers, these properties make the material promising for transparent electrode applications.
- Two-dimensional antimonene single crystals grown by van der Waals epitaxy,
Jianping Ji, Xiufeng Song, Jizi Liu, Zhong Yan, Chengxue Huo, Shengli Zhang, Meng Su, Lei Liao, Wenhui Wang, Zhenhua Ni, Yufeng Hao, Haibo Zeng,
Nat. Commun. 2016, 7, 13352.
DOI: 10.1038/ncomms13352