Iron’s most stable oxidation states in nature are +2 and +3. It only occurs as high-valent iron in reactive species involved in catalysis or in enzymes with a heme cofactor. These enzymes have inspired the synthesis of a number of biomimetic iron(IV,V) complexes. Nonbiomimetic high-valent iron complexes, in contrast, are rare and usually unstable in polar solvents such as water.
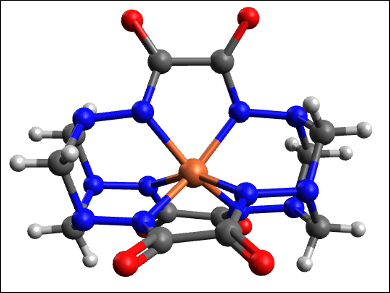
Igor O. Fritsky, Taras Shevchenko National University of Kyiv, Ukraine, and colleagues have synthesized iron(IV) complexes that are stable at ambient conditions in water. They are also the first examples of high-valent iron complexes formed spontaneously by air oxidation. Usually, low-valent iron species do not react with atmospheric O2 at ambient conditions. The team combined iron(III) nitrate, oxalodihydrazide (oxh), and formaldehyde in an alkaline aqueous solution to form a cage-like iron(IV) hexahydrazide clathrochelate complex (pictured).
The isolated complex is stable both in solution and in the solid state, with no change in its Mössbauer spectrum even after one year. According to the researchers, the ligand stabilizes the high oxidation state of iron by strong electron donation and shielding. The complexes could potentially be used in redox catalysis.
- Indefinitely stable iron(IV) cage complexes formed in water by air oxidation,
Stefania Tomyn, Sergii I. Shylin, Dmytro Bykov, Vadim Ksenofontov, Elzbieta Gumienna-Kontecka, Volodymyr Bon, Igor O. Fritsky,
Nat. Commun. 2017, 8, 14099.
DOI: 10.1038/ncomms14099