Polyhedral boron clusters, for example, dodecaborate (B12H122–), can be stable under harsh conditions such as strong acidity or high temperatures. By functionalizing such compounds, their properties could be tuned for a range of applications, e.g., as photoactive materials, catalysts, or diagnostic agents. The smaller hexaborate cluster B6H62– is less well studied than the B12-based clusters. It has a nucleophilic character and has previously been partially substituted using carbon-based electrophiles. However, it had not been peralkylated so far.
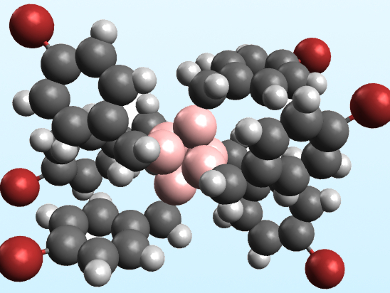
Alexander M. Spokoyny, University of California, Los Angeles, USA, and colleagues have synthesized the first perfunctionalized hexaborate cluster featuring B−C bonds. The team reacted [NBu4][B6H6Hfac] (Hfac = proton on the cluster face) with benzyl bromide and Hünig’s base (N,N-diisopropylethylamine) in acetonitrile under microwave heating. The product was the peralkylated cluster [NBu4][B6(CH2C6H5)6Hfac], which was characterized using NMR spectroscopy. The team also synthesized the analogous cluster [NBu4][B6(CH2C6H4Br)6Hfac] (pictured) using 4-bromobenzyl bromide, which formed crystals that could be characterized using X-ray crystallography.
The synthesized compounds are stable under ambient conditions. The team suggests that this metal-free alkylation reaction is driven by the nucleophilic character of the hexaborate anion. Based on this approach, hexaborate clusters could potentially be functionalized using a range of other electrophiles.
- Metal-Free Peralkylation of the closo-Hexaborate Anion,
Jonathan C. Axtell, Kent O. Kirlikovali, Dahee Jung, Rafal M. Dziedzic, Arnold L. Rheingold, Alexander M. Spokoyny,
Organometallics 2017.
DOI: 10.1021/acs.organomet.7b00078