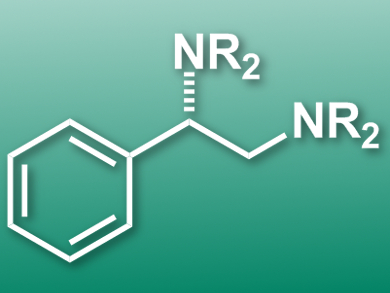
Vicinal diamines are highly important in the biomedicinal and pharmaceutical fields. The direct diamination of alkenes is a convenient approach for their synthesis. However, diamines are effective bidentate ligands and strongly coordinate transition metals. Therefore, it is difficult to find metal catalysts for their synthesis. Homogeneous iodine-based redox catalysis is a promising alternative.
Kilian Muniz, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, and colleagues have developed an iodine(I/III)-catalyzed enantioselective diamination of styrenes under intermolecular reaction control to give vicinal diamines. The deamination relies on a chiral aryliodine(I) catalyst and uses 3-chloroperbenzoic acid as a terminal oxidant. The approach uses an environmentally friendly solvent combination (tBuOMe/hexafluoro-2-propanol), which also slows down the rate of undesired background reactions such as epoxidations. High enantiomeric excesses in the range 91−98 % were achieved for a total of 30 examples.
According to the researchers, the approach is the first example of an iodine(I/III)-catalyzed enantioselective version of the reaction and could be used to synthesize a range of alkaloids and pharmacophores.
- Catalytic Asymmetric Diamination of Styrenes,
Kilian Muniz, Laura Barreiro, R. Martín Romero, Claudio Martínez,
J. Am. Chem. Soc. 2017.
DOI: 10.1021/jacs.7b01443