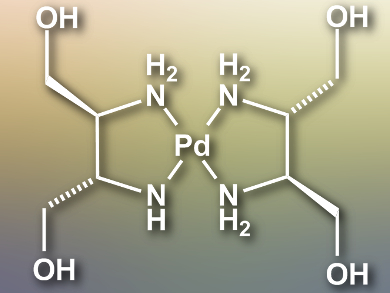
The chemistry of palladium(III) compounds has attracted researchers’ attention because of their potential for solid-state functional materials. However, the thermal and air instability of known Pd(III) complexes has prevented further detailed studies.
Hiroaki Iguchi, Masahiro Yamashita, Tohoku University, Sendai, Japan, and colleagues have synthesized a bromide-bridged Pd(III) chain complex [Pd(dabdOH)2Br]Br2 (Pd(dabdOH)2 moiety pictured). The ligand (2S,3S)-2,3-diaminobutane-1,4-diol (dabdOH) is synthesized from L-(+)-tartaric acid and reacts with PdBr2 in water at 55 °C to give [Pd(dabdOH)2]Br2. The desired Pd(III) complex is synthesized by slowly diffusing Br2 vapor into the [Pd(dabdOH)2]Br2 solution.
The large in-plane dabdOH ligand forms a multiple-hydrogen-bond network. This shortens the Pd–Br–Pd distance and stabilizes the Pd(III) oxidation state up to a decomposition temperature of 443 K. The complex is the most conductive MX-type chain complex to date, with a conductivity of 3–38 S cm–1 at room temperature. This study shows that the electronic states, thermal stability, and conductivity of linear coordination polymers can be controlled by precisely controlling the position of ions with a multiple-hydrogen-bond network.
- Multiple-Hydrogen-Bond Approach to Uncommon Pd(III) Oxidation State: A Pd–Br Chain with High Conductivity and Thermal Stability,
Mohammad Rasel Mian, Hiroaki Iguchi, Shinya Takaishi, Hideaki Murasugi, Tatsuya Miyamoto, Hiroshi Okamoto, Hisaaki Tanaka, Shin-ichi Kuroda, Brian K. Breedlove, Masahiro Yamashita,
J. Am. Chem. Soc. 2017, 139, 6562–6565.
DOI: 10.1021/jacs.7b02558