n-Butyllithium (n-BuLi) is a widely used reagent in organic synthesis. Organolithium reagents such as n-BuLi can form aggregates or solvent complexes in solution, which can affect their reactivity. They can be studied using, e.g., 6Li NMR spectroscopy. However, even at very low temperatures (–80 °C), the observed 6Li peaks of n-BuLi overlap using this method and thus, the structure of its aggregates cannot be determined.
Paul G. Williard and colleagues, Brown University, Providence, RI, USA, have used diffusion-ordered spectroscopy (DOSY) NMR techniques to study the aggregation and solvation of n‑BuLi in hydrocarbon solvents and in ethers such as tetrahydrofuran (THF) or diethyl ether. DOSY techniques allow the separation of NMR signals of different chemical species. The team monitored the solvation process by gradually adding an ether solvent to solutions of n-BuLi in a hydrocarbon solvent (so-called NMR titration). They also studied the influence of temperature on aggregates of n-BuLi.
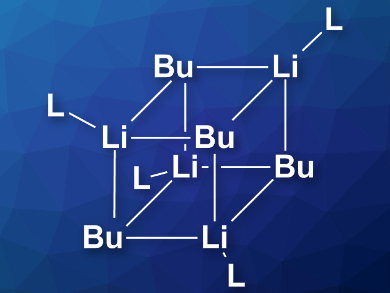
The researchers found that n-BuLi mostly exists as an octamer in hydrocarbon solvents (hexanes) at –40 °C. When the temperature is increased, a hexamer aggregate becomes predominant. After adding THF or diethyl ether, the most common species is a tetramer with four coordinated solvent molecules (pictured). If the concentration of ether is raised, this tetramer partially deaggregates and a tetra-solvated dimer is formed. According to the researchers, this information can provide insight into the reactivity difference of n-BuLi under different circumstances.
- Aggregation and Solvation of n-Butyllithium,
Onkei Tai, Russell Hopson, Paul G. Williard,
Org. Lett. 2017.
DOI: 10.1021/acs.orglett.7b01644