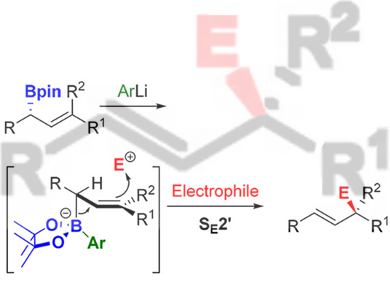
The reaction of allylmetals and electrophiles is one of the most important reactions in organic synthesis. Allylborons are ideal nucleophilic reagents because of their configurational stability, easy accessibility, and inertness towards 1,3-transposition, but their application is severely limited by their low nucleophilicity. Although their reactions with carbonyls and imines are well developed and widely used, the reactions with other electrophiles are virtually unknown.
Varinder K. Aggarwal, University of Bristol, UK, and colleagues have developed an effective approach to enhance the nucleophilicity of allylboronates. A highly reactive and configurational stable boronate complex is formed by adding boronic esters to in-situ-generated aryllithium in THF at –78 °C. This complex can react regio- and stereoselectively with a broad range of carbon- and heteroatom-based electrophiles, such as tropylium, activated pyridines, Eschenmoser’s salt, Togni’s reagent, Selectfluor, dimethyl(methylthio)sulfonium, etc.
According to the researchers, the method increases the nucleophilicity of the boronic ester by 7 to 10 orders of magnitude and provides a new possibility to synthesize the compounds that are otherwise difficult to obtain.
- Stereospecific Allylic Functionalization: The Reactions of Allylboronate Complexes with Electrophiles,
Cristina Garcia-Ruiz, Jack L.-Y. Chen, Christopher Sandford, Kathryn Feeney, Paula Lorenzo, Guillaume Berionni, Herbert Mayr, Varinder K. Aggarwal,
J. Am. Chem. Soc. 2017.
DOI: 10.1021/jacs.7b10240