Metal corrosion is an important and often costly problem in many branches of industry. Standard anticorrosion coatings need to be relatively thick. Graphene, in contrast, could prevent oxygen and water from reaching a metal surface with only a negligible coating thickness. However, it is semi-metallic and could even accelerate electrochemical corrosion by forming a circuit with the metal. These two contradictory properties need to be better understood to develop useful graphene coatings for metals.
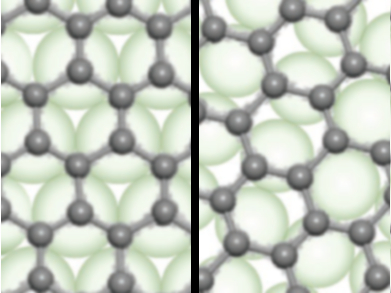
Ying Jiang and Kaihui Liu, Peking University and Collaborative Innovation Centre of Quantum Matter, both Beijing, China, Feng Ding, Institute for Basic Science and Ulsan National Institute of Science and Technology (UNIST), both Ulsan, Republic of Korea, and colleagues have studied graphene-coated copper using corrosion experiments, scanning tunnelling microscopy (STM), and scanning tunnelling spectroscopy (STS). The team compared graphene-coated copper(111) surfaces (pictured left, in green) and graphene-coated copper(100) surfaces (pictured right, in green) using these methods.
The researchers found that both surfaces can be protected from O2 by graphene. However, only Cu(111) surfaces are protected from corrosion in the presence of water, while Cu(100) surfaces are heavily oxidized under water vapor. The team attributes this difference to the interactions between graphene and the copper surfaces: While the graphene lattice is well aligned with Cu(111) (pictured left), it does not match the structure of Cu(100) surface (pictured right). This causes creases in the graphene layer that allow H2O diffusion to the metal surface. According to the researchers, these insights could allow the use of graphene for large-scale and long-term anticorrosion applications by applying it to suitable metal surfaces.
- Greatly Enhanced Anticorrosion of Cu by Commensurate Graphene Coating,
Xiaozhi Xu, Ding Yi, Zhichang Wang, Jiachen Yu, Zhihong Zhang, Ruixi Qiao, Zhanghao Sun, Zonghai Hu, Peng Gao, Hailin Peng, Zhongfan Liu, Dapeng Yu, Enge Wang, Ying Jiang, Feng Ding, Kaihui Liu,
Adv. Mater. 2017.
https://doi.org/10.1002/adma.201702944