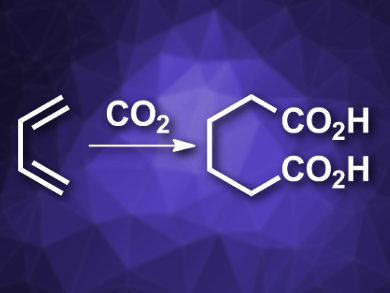
Carbon dioxide is both a pollutant and a useful chemical feedstock. It can, e.g., be inserted into double bonds in carboxylation reactions. However, the insertion of multiple CO2 molecules into a molecule has been difficult to realize so far.
Ruben Martin, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, and Catalan Institution for Research and Advanced Studies (ICREA), Barcelona, Spain, and colleagues have developed a mild, selective, nickel-catalyzed reaction protocol which can be used to insert two molecules of CO2 into two C=C double bonds. The reaction converts 1,3-dienes to dicarboxylic acids (pictured). The team combined a diene with NiBr4(TBA)2 (TBA = tetrabutylammonium) as a catalyst, a phenanthroline derivative as a ligand, and Mn as a reductant in dimethylacetamide (DMA) under 1 atm of CO2 at 50 °C.
The reaction gives the desired adipic acid derivatives in good yields. The protocol is regio- and chemoselective and tolerates a variety of functional groups. It proceeds under mild conditions and uses readily available precursors, which are produced on a large scale as byproducts of steam cracking in the production of ethylene.
- Ni-Catalyzed Site-Selective Dicarboxylation of 1,3-Dienes with CO2,
Andreu Tortajada, Ryo Ninokata, Ruben Martin,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.7b13220