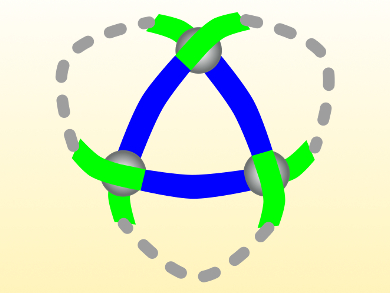
The trefoil knot, named after the three-leaf clover plant, has three crossings and is the simplest knot that cannot be untied without a cut. Twisting small molecules into this shape is challenging, and usually involves strategies that cannot be transferred to other molecular knots.
David A. Leigh and colleagues, University of Manchester, UK, have synthesized a molecular trefoil knot using a ring-closing reaction between the “loose” ends of a trimeric circular helicate (pictured). Circular helicates with multiple metal ions have previously been used as scaffolds for building molecules with four to eight crossings.
The team combined pyrazine-2,5-dicarbaldehyde, a 2-(2-aminoethyl)pyridine derivative with a terminal alkene group, and Zn(BF4)·H2O to form the helicate intermediate. In this step, the dicarbaldehyde (pictured blue) reacts with two equivalents of the amine (pictured green) to form strands. Three of these strands self-assemble into the helicate, where they are interconnected by three zinc ions at the crossings. The helicate was converted to the closed knot by ring-closing metathesis between the terminal alkene groups using a Hoveyda−Grubbs second generation catalyst.
The knot features a loop containing 84 atoms and was prepared in 90 % yield in only two steps. According to the researchers, this modular approach could be transferable to other synthetic targets and allow the synthesis of more complex molecular knots.
- Molecular Trefoil Knot from a Trimeric Circular Helicate,
Liang Zhang, David P. August, Jiankang Zhong, George F. S. Whitehead, Iñigo J. Vitorica-Yrezabal, David A. Leigh,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b00738