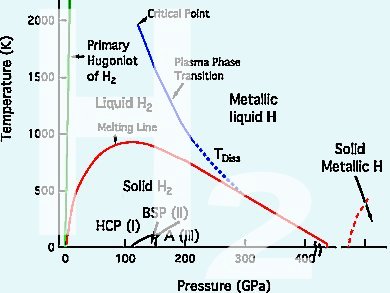
Eugene Gregoryanz, University of Edinburgh, UK, and colleagues discovered a new phase of hydrogen. In this phase IV, which occurs at very high pressures, the diatomic molecules break apart to form six-atom rings, similar to graphene. This could be a stepping stone towards metallic hydrogen which for example has the potential for applications like room temperature superconductors, which would enable lossless power transmission.
So far there have been three known solid phases of hydrogen which can be created by supercooling the gas:
- phase I: close-packed structure of freely rotating molecules;
- phase II: similar structure but with some degree of orientational order;
- phase III: structure in which the strength of the H–H bonds turns so weak that the hydrogen could be considered partly atomic, not molecular.
The researchers used Raman and visible transmission spectroscopy to investigate dense hydrogen and deuterium up to 315 and 275 GPa, respectively, at 300 K. They measured the vibron frequency, which determines the strength of the H–H bonds and which therefore describes how ‘molecular’ the hydrogen is.
At 220 GPa the main vibron frequency rapidly decreased due to the formation of graphene-like layers of hydrogen in irregular six-atom rings in phase IV. At the same time a second vibron appeared, maintaining the original frequency which relates to the graphene-like layers being interspersed with unbound hydrogen molecules.
Image: ©: PNAS, National Academy of Sciences
- Mixed Molecular and Atomic Phase of Dense Hydrogen,
Ross T. Howie, Christophe L. Guillaume, Thomas Scheler, Alexander F. Goncharov, Eugene Gregoryanz,
Phys. Rev. Lett. 2012, 108, 125501.
DOI: 10.1103/PhysRevLett.108.125501