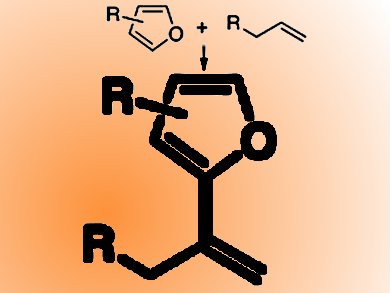
Vinylarenes can be formed via the Mizoroki−Heck reaction, but this requires functionalized coupling partner.
John F. Hartwig and Christo S. Sevov, University of California, Berkeley, CA, USA, report an attractive oxidative coupling of arenes and alkenes to synthesize vinylarenes. This olefination of furans with unactivated alkenes, including unstrained internal alkenes and propene, is catalyzed by Ir and a second alkene acts as hydrogen acceptor. The reaction resulted in excellent yields and occurred with high selectivities.
The team suggests that this reaction occurs by turnover-limiting migratory insertion of an alkene into an Ir−C bond whereby the product formes faster at low temperatures than at higher temperatures. New ligands were developed that form complexes capable of catalyzing the olefination of a wide range of furans under mild conditions.
The researchers hope to extend this process to arenes.
- Iridium-Catalyzed Oxidative Olefination of Furans with Unactivated Alkenes,
Christo S. Sevov, John F. Hartwig,
J. Am. Chem. Soc. 2014.
DOI: 10.1021/ja504414c