Researchers at the Humboldt-Universität zu Berlin, Germany, and colleagues at the Technische Universität Berlin and Brookhaven National Laboratory in the USA, explain how the development of new and more effective catalysts invariably hinges on an improved understanding of the intermediates formed by the catalyst as it works on the reagents.
Chemists had previously suggested that certain copper-catalyzed reactions might involve high-valent, terminal copper–nitrene units in aziridination and amination reactions but these species had remained elusive for decades ensuring that the mechanism of such reactions remained hidden.
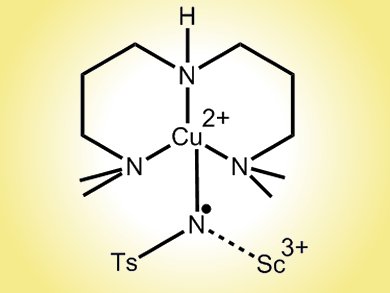
Now, the international team has used a Lewis acid, scandium triflate, at –90 °C, to trap one such intermediate, a copper–tosylnitrene. The research suggests that Cu(III)NTs – an unusual oxidation state for the metal two levels above copper(I) – are the core component of the copper-based oxidation catalysis used in aziridination and amination reactions. The work may also provide insights into the catalytic cycle of mononuclear copper monooxygenases.
- Lewis Acid Trapping of an Elusive Copper–Tosylnitrene Intermediate Using Scandium Triflate,
Subrata Kundu, Enrico Miceli, Erik Farquhar, Florian Felix Pfaff, Uwe Kuhlmann, Peter Hildebrandt, Beatrice Braun, Claudio Greco, Kallol Ray,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja306674h