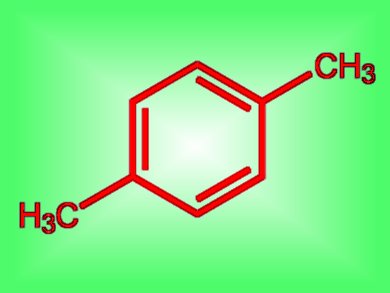
As the price of oil goes up and supplies dwindle, alternative feedstocks become increasingly important for servicing the chemical industry. Ethylene, commonly released from oil industrially, can also be generated from biomass via bioethanol. A team at the University of North Carolina at Chapel Hill, USA, have demonstrated that ethylene can act as a useful feedstock in the preparation of an important commodity chemical, p-xylene (pictured). This chemical is one of the highest volume chemicals derived from petroleum. It is used to generate the dimethyl ester of terephthalic acid for copolymerization with ethylene glycol to produce 11 billion tonnes of polyethylene terephthalate (PET) annually.
p-Xylene is one of the products of catalytic reforming of various crude oil streams. However, the team has shown how ethylene, as the sole feedstock, can be trimerized to hexane and thence to 2,4-hexadiene. A Diels-Alder reaction with another ethylene molecule then forms 3,6-dimethylcyclohexene. p-Xylene can then be produced from this intermediate without isomeric side-products through catalytic dehydrogenation, the team explains.
- Synthesis of p-Xylene from Ethylene,
Thomas W. Lyons, Damien Guironnet, Michael Findlater, Maurice Brookhart,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja307612b