The structure and dynamics of water films on metallic surfaces has received growing attention in the past two decades, as water-metal interactions play a central role in many catalytic surface reactions, corrosion processes, and electrochemistry. So far, little is known about the chemistry of water on stepped surfaces, although these are commonly occurring realistic situations.
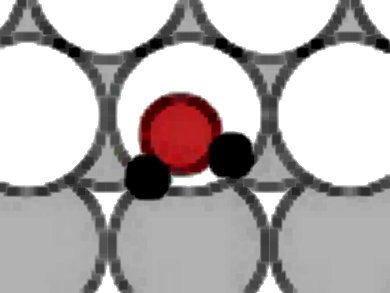
Davide Donadio, Max Planck Institute for Polymer Research, Mainz, Luca M. Ghiringhelli, Fritz-Haber-Institut, Berlin-Dahlem, and Luigi Delle Site, Freie Universität Berlin, all Germany, used first-principles simulations to study the adsorption of water on a stepped platinum surface. Water adsorbs preferentially at the step edge, forming linear clusters or chains. These are stabilized by the cooperative effect of chemical bonds with the substrate and hydrogen bonds.
In contrast to their behavior on flat Pt, at steps water molecules dissociate, forming mixed hydroxyl/water structures, through an autocatalytic mechanism promoted by H-bonding. Nuclear quantum effects contribute to stabilize partially dissociated cluster and chains. Partial dissociation occurs only when the coverage is sufficient to produce trimers or larger clusters, and is promoted by the combination of O−Pt interaction and hydrogen-bonding.
Spontaneous partial dissociation of water wires makes them viable ionic conductors, since the barriers for proton hopping along hydrogen-bonded chains were shown to be relatively low, at most 0.15 eV. Conduction would occur via diffusion of proton vacancies (holes).
- Autocatalytic and Cooperatively Stabilized Dissociation of Water on a Stepped Platinum Surface,
Davide Donadio, Luca M. Ghiringhelli, Luigi Delle Site,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja308899g