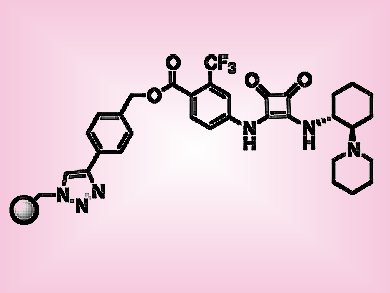
The popularity of supported catalysts in organic synthesis has increased significantly in recent years. Immobilized catalysts are easy to recover and reuse, and may be suited to continuous flow processes. In particular, chiral organocatalysts lend themselves to this type of reaction because they are robust and work without metal cofactors, which may be leached.
Miquel Pericàs, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, and colleagues studied the Michael addition reaction of 2-hydroxy-1,4-naphthoquinone with a series of nitroalkenes, catalyzed by means of a polystyrene-supported bifunctional squaramide organocatalyst (pictured). The reactions were fast, typically 2 h, highly enantioselective (>91 % e.e.) and required low catalyst loadings (2 mol %). Because the reactions cleanly resulted in the desired products, production under continuous flow conditions was explored. The catalyst could be recycled ten times without any decrease in enantioselectivity (average 96 % e.e.) and adapted to continuous flow operation (24 h). In addition, six different nitroalkenes were reacted sequentially, which highlights the suitability of this method for preparing focused libraries of use to medicinal and materials chemists.
- Continuous Flow, Highly Enantioselective Michael Additions Catalyzed by a PS-Supported Squaramide,
Pinar Kasaplar, Carles Rodríguez-Escrich, Miquel A. Pericàs,
Org. Lett. 2013.
DOI: 10.1021/ol400974z