H. V. M. Hamelers and colleagues, Wetsus, Centre of Excellence for Sustainable Water Technology, Leeuwarden, the Netherlands, harvested the mixing energy from CO2-containing gas emissions.
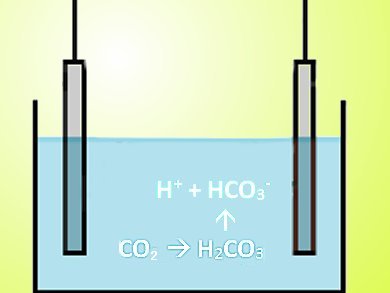
CO2 was mixed with a liquid, using either deionized water or a 0.25 M water solution of monoethanolamine (MEA), which is often used to remove CO2 from exhaust gases. In water, the CO2 forms carbonic acid, which dissociates into H+ and HCO3–. A current between the electrodes is produced as the ion-exchange membranes on the cell’s electrodes absorb the ions.
Then mostly ion free water with dissolved fresh air is flushed through the cell. The membranes release the ions into the water and produce current in the opposite direction as before. The ion-laden water leaves the cell and gets flushed with air. CO2 reforms and is then released. The fluidics system continually repeats this cycle.
An efficiency of 24 % was reached with deionized water as the aqueous electrolyte and 32 % with a 0.25 M MEA solution as the electrolyte. The highest average power density obtained with a MEA solution was 4.5 mW/m2, but with water as the electrolyte only on of 0.28 mW/m2.
According to the researchers with a scaled-up system, power plants could produce megawatts of power using CO2 emissions. Flue gases from power plants worldwide contain enough CO2 to generate 850 TWh of energy every year, they say.
However, a problem to be solved are to get enough CO2 emissions dissolved into the liquid without requiring more energy than the system could generate and to prevent impurities in a power plant’s flue gas, such as sulfur dioxide or nitrogen oxides, from fouling the cell’s membranes.
- Harvesting Energy from CO2 Emissions,
H. V. M. Hamelers, O. Schaetzle, J. M. Paz-García, P. M. Biesheuvel, C. J. N. Buisman,
Environ. Sci. Technol. Lett. 2013.
DOI: 10.1021/ez4000059