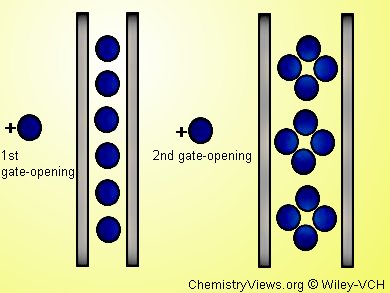
To selectively capture molecules possessing an electron-acceptor character like nitric oxide, Hitoshi Miyasaka and colleagues, RIKEN Spring-8 Center, Hyogo, Japan, have synthesized one-dimensional chain compounds possessing a high donor character. They used 4-chloroanisate-bridged paddlewheel-type dimetal(II, II) complexes with M = Ru and Rh and phenazine (phz) as the chain linker: [M2(4-Cl-2-OMePhCO2)4(phz)]•n(CH2Cl2).
CH2Cl2 occupies the spaces between chains of [−{M2}−phz−] repeating units, until NO guest molecules trigger stepwise transformations with hysteresis absorption/desorption behavior: in a first gate-opening absorption step, NO is physisorpt by the gas-recognition ability of the pores. In a second gate-opening absorption step host/guest chemical interactions occur.
A charge transfer between the host [−{M2}−phz−] framework and the NO molecules plays a key role for the specific adsorption behavior. The phz unit, which is activated by a π back-donation mechanism from the [M2] units, and its near-field region, trap the NO molecules involving charge transfer.
The team thinks that the fine-tuning of the electrondonating ability of framework components is a promising strategy to achieve the advanced selective absorption properties.
- Selective NO Trapping in the Pores of Chain-Type Complex Assemblies Based on Electronically Activated Paddlewheel-Type [Ru2II,II]/[Rh2II,II] Dimers,
Wataru Kosaka, Kayo Yamagishi, Akihiro Hori, Hiroshi Sato, Ryotaro Matsuda, Susumu Kitagawa, Masaki Takata, Hitoshi Miyasaka,
J. Am. Chem. Soc. 2013.
DOI: 10.1021/ja4076056