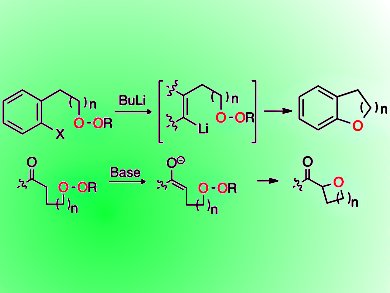
Critical substructures in many bioactive molecules and natural products are ethers. They are typically synthesized through attack of a nucleophilic oxygen on an electrophilic carbon.
Rachel Willand-Charnley, Benjamin W. Puffer, and Patrick H. Dussault, University of Nebraska—Lincoln, USA, have demonstrated a new approach to synthesize spirocyclic ethers, aryl ethers, and various oxacycles including oxetanes.
They generated carbanions via chemoselective metal-heteroatom exchange or deprotonation. An intramolecular reaction of dialkyl peroxides with the carbanios leads to cyclic ethers. Applied in tandem with C−C bond formation, the strategy enables a one-step annelation to form oxaospirocycles.
- Oxacycle Synthesis via Intramolecular Reaction of Carbanions and Peroxides,
Rachel Willand-Charnley, Benjamin W. Puffer, Patrick H. Dussault,
J. Am. Chem. Soc. 2015.
DOI: 10.1021/ja5026276



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)