The fission of uranium in nuclear power plants generates radionuclides such as 137Cs and 90S. These isotopes have halflives of roughly 30 years. Selectively removing them from nuclear waste could facilitate safe storage until their activity has decreased sufficiently.
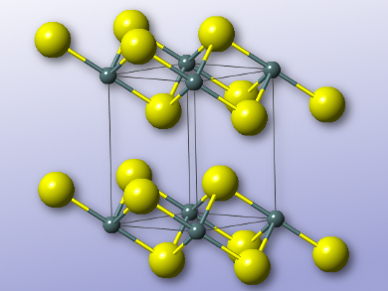
Mercouri G. Kanatzidis, Northwestern University, Evanston, USA, and colleagues have developed the layered sulfide K2xSn4−xS8−x (x = 0.65–1). The team synthesized the compound by heating potassium carbonate, tin powder, and elemental sulfur in a hydrothermal process at 220 °C for 15 hours. The resulting sulfide was characterized by X-ray crystallography, as well as IR and Raman spectroscopy. It crystallizes in the space group P21/c, and consists of anionic layers composed of SnS6 octahedra and SnS4 tetrahedra, with potassium cations between the layers.
The interlayer potassium ions can be exchanged for Cs+, Sr2+, or UO22+ ions rapidly, capturing the radionuclides. The researchers suggest further investigations into the use of the material for nuclear waste storage.
- K2xSn4−xS8−x (x = 0.65–1): a new metal sulfide for rapid and selective removal of Cs+, Sr2+ and UO22+ ions,
Debajit Sarma, Christos D. Malliakas, K. S. Subrahmanyam, Saiful M. Islam, Mercouri G. Kanatzidis,
Chem. Sci. 2016.
DOI: 10.1039/C5SC03040D